A keyhole that changes shape A bifunctional enzyme rewrites the paradigm of the monofunctional enzyme

Researchers at the University of Tokyo (Todai) have shown conclusively and for the first time that one enzyme changes shape to catalyze two reactions.
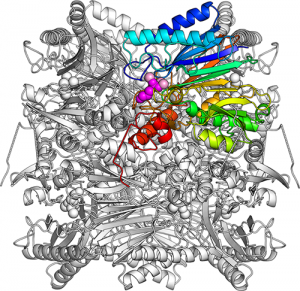
The overall structure of FBPA/P. Eight identical subunits are closely packed together forming a barrel-shape. One subunit is picked out in colors (blue, cyan, green, yellow, orange, red). An attached DHAP (dihydroxyacetone phosphate) molecule is shown as purple-colored spheres and associated magnesium ions are marked as pink-colored spheres.
Normally, enzymes have an active site formed to perfectly fit a specific molecule and catalyze a single reaction?like a keyhole that accepts only one specific key. But there is a unique enzyme that catalyses two reactions at one active site. Fructose-1,6-bisphosphate (FBP) aldolase/phosphatase (FBPA/P) is a primordial enzyme that catalyses both aldol condensation to create FBP and dephosphorylation of FBP fructose-6-phosphate (F6P). How it achieved this was unknown.
Professor Takayoshi Wakagi, Associate Professor Shinya Fushinobu, Project Assistant Professor Hiroshi Nishimasu and their research team at Todai’s Graduate School of Agricultural and Life Sciences have shown that FBPA/P adjusts the shape of its active site to catalyze each reaction in turn.
The team analyzed the crystal structure of FBPA/P from a thermoacidophilic archaeon and succeeded in determining its form in the aldolase reaction. Comparing this with a previously determined phosphatase form, they showed that FBPA/P dramatically changes the shape of its active site.
The current concept of enzymes that one active site catalyzes one reaction needs to be expanded to include the concept of bifunctional enzymes. FBPA/P is responsible for gluconeogenesis, which was probably of critical importance in the early stages of evolution in an environment poor in life-sustaining materials. Their findings suggest the possible existence of undiscovered enzymes capable of catalyzing multiple reactions on single active sites, and also enzymes capable of creating useful compounds from simple starting materials.
Department release/press release (Japanese)
Paper
Shinya Fushinobu, Hiroshi Nishimasu, Daiki Hattori, Hyun-Jin Song, Takayoshi Wakagi,
“Structural basis for the bifunctionality of fructose-1,6-bisphosphate aldolase/phosphatase”
Nature 478, 538?541. doi:10.1038/nature10457
Article link






