Crystalline sponge method X-ray Crystallography without the Need for Crystallization

Single crystal X-ray analysis (X-ray crystallography) is one of the most reliable methods for determining molecular structure, but preparing crystals sufficient for analysis can be a severe bottleneck. Professor Makoto Fujita and his co-workers have developed a crystalline sponge method in which the crystallization step is rendered unnecessary. This new method is expected to revolutionize molecular structure determination.
“Seeing” Molecules with X-rays
The biggest frustration for chemists is the inability to directly see the molecules that are the target of their research. All that chemists are able to do is to collect data by applying magnetic or electric fields and light and estimating the molecular structures based on that data. In recent years, rapid progress has been made in electron microscopy, but the resolution is not yet sufficient to discuss molecular structure in fine detail. In short, a molecular structure drawn by a chemist is not actually a result of “seeing” the molecule, but is rather an image derived indirectly from various data.
X-ray crystallography is a reliable method for determining molecular structure at high resolution. In this method, accurate molecular structures can be obtained by analyzing the diffraction patterns of single crystal samples. Currently, it is the most reliable technique for structure analysis and can be said to be the closest thing to actually “seeing” a molecule.
The sore point of X-ray crystallography is the absolute necessity of obtaining a near perfect crystal. A crystal is a material in which the constituent compound is precisely assembled in three dimensions. If the target compound is not a single crystal, its molecular structure cannot be analyzed by X-ray crystallography. There are however, a great number of molecules that because of their particular characteristics generally do not crystallize. For example, compounds with flexible, spaghetti-like structures have difficulty assembling in regular formations, and form only oils or amorphous solids. Years can be spent unsuccessfully trying to obtain a high quality crystal, which can be a great drag on research.
Enter: the Crystalline Sponge Method
A great breakthrough in a discipline often appears from an entirely unexpected field. An idea for solving a problem that has perplexed crystallographers for the last century sprang from an area that at first glance is completely unrelated: macromolecular chemistry.
Professor Makoto Fujita of the Department of Applied Chemical Engineering in the Graduate School of Engineering, a noted scientist in the field of macromolecular chemistry, has made substantial achievements in research, particularly in self-assembly (the spontaneous assembly of simple molecular units into complex systems).
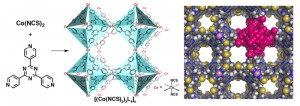
Professor Fujita and his co-workers discovered that metal ions and certain organic molecules could self-assemble to form a three dimensional lattice, and spent many years researching this phenomenon. These crystalline structures form regular, systematic cavities in the interior, creating a porous, sponge-like lattice.
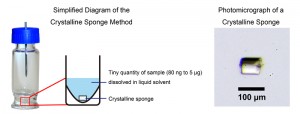
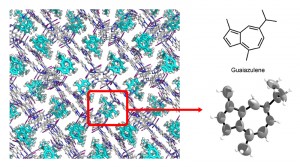
If a crystalline sponge is soaked in a solution containing a compound of interest, the compound is absorbed into the cavities and the molecules arrange themselves in a fixed orientation. If this is then analyzed by X-ray crystallography, the structure of the compound can be cleanly elucidated. Without relying on luck while going through the time-consuming process of obtaining a suitable crystal, the structure of an extremely small quantity of sample, as little as tens of nanograms, can be elucidated. This discovery, published in 2013 in the science journal Nature, and hailed by both domestic and international journals as a revolutionary analytical technique, has made a great impact on the scientific world.
The crystalline sponge method does not wait for randomly oriented molecules to spontaneously arrange in a neat order, but rather works on the very simple idea that the molecules merely need to enter the cavities of the lattice, divided like separate rooms in an apartment building. This crystalline grid material is known as a metal-organic framework (MOF), recently a rapidly evolving field of research worldwide.
Nevertheless, that the Fujita research group is the sole laboratory to finally realize this development speaks to the accumulated achievements of twenty years of research. The understanding of intermolecular interaction, many years of experience with X-ray crystallography, command of the methods of organic synthesis to design and prepare desired compounds. It is all of these accumulated high level technologies and knowledge that has made this achievement possible.
Room still remains to develop and improve this new crystalline sponge method. The day that anyone can easily elucidate a structure from a very small quantity of compound it will be possible to say that a revolution has been brought about in structural analysis. Their efforts continue towards that day.
Text: Kentaro Sato (science writer). Translation: Rader Jensen.
Links
Graduate School of Engineering
Department of Applied Chemistry, Graduate School of Engineering
Fujita Laboratory, Department of Applied Chemistry, Graduate School of Engineering