Aiming for a future without tangles The race to prevent Alzheimer's disease
As the mechanism of Alzheimer’s disease is better understood, it has become clear that early treatment is vital to prevent permanent damage to the brain. But even though potential treatments are emerging, without any way of measuring the progress of the disease in its early stages, there is no way to measure their effectiveness. The University of Tokyo is playing a central role in the large-scale research project J-ADNI, which aims to identify biomarkers to track the progress of Alzheimer’s disease from its onset.
Towards a World without Alzheimer’s Disease
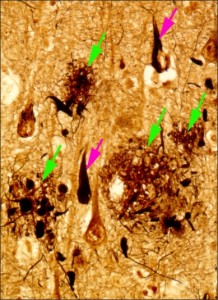
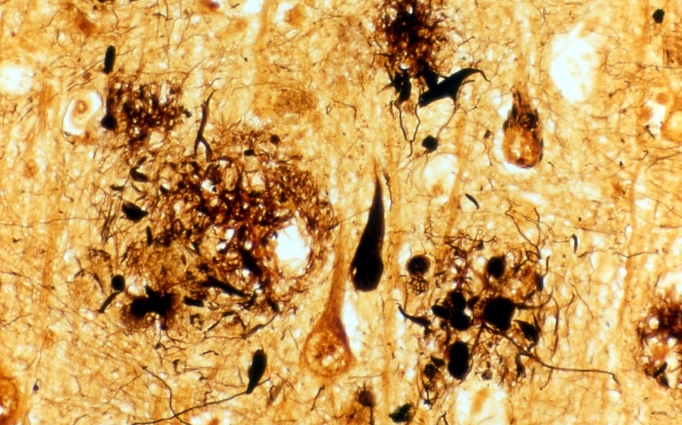
Figure 1: Brain tissue of an AD patient
Black areas are dead nerve cells, red arrows indicate string-like depositions of tau protein, green arrows indicate amyloid-β placques.
© Takeshi Iwatsubo.
As Japanese society ages, the number of dementia patients is on the rise. Their number is expected to reach some 3.25 million by 2020, making the search for diagnostic tools and treatments a pressing issue. Dementia is commonly defined as a chronic impairment or loss of cognitive function, not present at birth, that interferes with normal daily life and social interaction. Its most common cause is Alzheimer’s disease, AD for short.
Alzheimer’s disease is named after the German neuropathologist Dr. Alois Alzheimer who in 1906 first described it as a shrinking of the cerebrum, causing problems such as memory loss and loss of judgment as it progresses. Until very recently, it was an intractable disease with neither cure nor definite means of diagnosis. However, the pathogenesis of AD is now better understood, giving rise to the possibility of discovering a cure and preventive measures. Professor Takeshi Iwatsubo of the University of Tokyo’s Graduate School of Medicine is employing both basic science research and clinical trials as he works towards the goal of “a world without Alzheimer’s.”
Elucidating the Cause of AD
After receiving his M.D. from the University of Tokyo, Dr. Iwatsubo worked as a neurologist treating patients with brain disease. During his residency in the 1980s, he also began research into AD at the University’s neuropathology lab while studying diagnostic methods and continued this work into the 1990s. In that period, technological advancement in protein and genetic analysis led to rapid developments in the field of molecular neuropathology. Dr. Iwatsubo’s mentor, Prof. Yasuo Ihara (currently professor at Doshisha University) discovered the AD-related tau protein in pathological changes, and the disease, which until then could only be observed through visible macroscopic changes such as the shrinking of the brain, could now be traced through changes on the molecular level.
Dr. Iwatsubo recalls, “The topic grew more and more fascinating, and when I was appointed to a post in the School of Pharmaceutical Sciences, I decided to go into research. I was no longer involved in clinical work so I was able to focus on research full-time, and since I was in constant contact with scientists trained in chemistry, it became easier to make progress in basic research.”
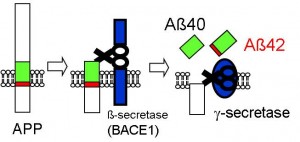
Figure 2: Formation process of amyloid-β
Amyloid precursor protein (APP) is cleaved into amyloid-β by the β-secretase and γ-secretase enzymes. First the β-secretase works on the outer cells and then the γ-secretase cleaves the APP in the cell membrane. Depending on where the cleaving occurs, either amyloid-β 40 or amyloid-β 42 is produced.
© Takeshi Iwatsubo.
It was already known that AD is triggered by an accumulation of amyloid-β protein, leading to string-like deposits of tau protein, which in turn results in the degeneration and loss of nerve cells and the shrinking of the brain. As genetic research progressed, it also became clear that amyloid-β is created when its precursor protein in the brain is cleaved by the enzyme γ-secretase, but the nature of this enzyme was still not understood. The commonly observed form of amyloid-β consists of a chain of 40 amino acids. However, Dr. Iwatsubo and his colleagues discovered depositions of amyloid-β consisting of chains of 42 amino acids in the brain tissue of AD patients. Amyloid-β 42 tends to accumulate more easily in the brain, and was found to play an important role in triggering the disease through actions such as promoting the deposition of tau protein.
“In 1995, presenilin was found to be the causative gene of familial Alzheimer’s disease, a type of AD. We then embarked on a series of experiments to determine whether this gene is linked to γ-secretase.” Dr. Iwatsubo, together with a graduate student in his laboratory, Dr. Taisuke Tomita (currently associate professor at the University of Tokyo’s Graduate School of Pharmaceutical Sciences) demonstrated that when the presenilin protein encoded by this gene is mutated, it results in the production of amyloid-β 42, which normally is formed at quite a low level. The accumulation of amyloid-β 42 in the brain then triggers the development of AD.
In first decade of the new century, as the technology for gene decoding advanced further, the analysis of γ-secretase progressed accordingly. As a result, it was established that γ-secretase consists of presenilin protein and three other types of proteins. First, two types of proteins form a complex with the presenilin. Then, with the addition of a protein called PEN-2, the active enzyme γ-secretase is formed. It also became clear that γ-secretase only becomes active when this construction sequence is maintained.
Large-Scale Clinical Trials Aimed at Early Diagnosis
As the pathogenic mechanism of AD became clear, the focus turned towards treatment and cure. Researchers searched for ways to prevent the accumulation of the disease-causing amyloid-β in the brain, or to eliminate it altogether. A variety of potential therapeutic drugs were developed, but there were as yet no standards by which the effectiveness of such drugs could be judged, for example pinpointing when and by how much the formation of amyloid-β must be suppressed in order for the drug to be deemed effective. Also, in the case of AD, amyloid-β will already have been accumulating in the brain for quite some time before symptoms start to appear. At the stage where a person is diagnosed with AD, changes in the brain have already progressed considerably. For treatment to be successful it is necessary to start as early as possible, before actual symptoms appear. But as yet there is no standard for the early diagnosis of AD, and it is not clear at which point treatment should begin. Consequently, even though potential drugs are being successfully developed, there is no way to determine their efficacy.
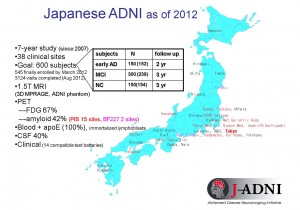
Figure 3: Outline of J-ADNI
Clinical trials are to be carried out over a 5-year period at 38 sites all over Japan.
© Takeshi Iwatsubo.
In 2005, ADNI (Alzheimer’s Disease Neuroimaging Initiative), a large-scale clinical study program, was launched in the United States with the aim of establishing benchmarks for the diagnosis and treatment of AD. Using image diagnosis technology and other advanced methods, changes in the brain from the normal state to the onset of the disease were carefully and thoroughly tracked in order to come up with biomarkers to pinpoint the various stages in the progress of AD.
“When I heard about this project from a research colleague at the University of Pennsylvania, I immediately thought that we must start a similar program in Japan as well. My colleagues were of the same opinion, and we started the preparatory work for a Japanese version of ADNI (J-ADNI).” In 2007, J-ADNI was officially launched with Dr. Iwatsubo serving as project leader and the participation of 38 institutions throughout Japan.
J-ADNI involves about 600 subjects who are being tracked over a period of two to three years through brain scans, biochemical, psychological and other tests. In order to enable eventual standardization of the diagnosis, the methods and evaluation parameters used for the project duplicate those of the US ADNI as closely as possible.
Dr. Iwatsubo points out that “the amyloid PET (Positron Emission Tomography) method allowing in vivo observation of amyloid-β accumulated in the brain was first implemented in Japan. J-ADNI has added these cutting-edge testing methods to the US model.” The Japanese amyloid PET method has now been added as a test item to the US ADNI as well. This first phase of J-ADNI will end in 2013, and the project has already yielded a great deal of information, including on the relationship between amyloid-β accumulation and the progress of dementia, contributing significantly to the development of diagnostic methods for AD.
Towards Early Treatment
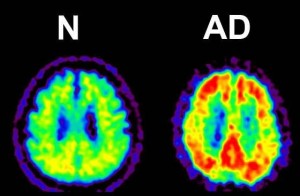
Figure 4: Amyloid PET (Positron Emission Tomography).
Amyloid-β accumulations are visible as yellow sections in this image amyloid PET image.
© Kenji Ishii.
Building on the results of J-ADNI, research into treatments with the goal of transitioning to full-fledged clinical trials began in 2013. In this project, new drugs will be tested on humans for the first time, to establish safety and determine the effectiveness of medication at a stage before the manifestation of clinical symptoms. The clinical trials for the project will be carried out at the University of Tokyo Hospital. Dr. Iwatsubo is enthusiastic: “In Japan, clinical trials are usually conducted by the pharmaceutical company developing the drugs. The current physician-led project is a first. With a view towards early treatment of Alzheimer’s disease, we want to establish a framework for treatment and diagnosis as quickly as possible. In addition, J-ADNI is also entering its second phase targeting preclinical AD, where amyloid has started to accumulate but no symptoms have yet appeared.”
“There are still many things we do not know about AD, and basic research is of course ongoing. But thanks to the results of J-ADNI, we have been able to bridge from basic to clinical research, and we are nearing our goal of applying the fruits of that research to clinical practice. In order to realize a world without AD, we need both strong determination and talented researchers. Besides specialists from the medical and pharmaceutical side, we must also involve experts from fields such as psychology and the cognitive and behavioral sciences.”
The US ADNI has also advanced to the second stage, and similar projects are being pursued in South Korea and in Europe. As a result of these efforts, a worldwide standard for the diagnosis of Alzheimer’s disease is likely to be formulated in the near future. The University of Tokyo aspires to be a major base of AD research, for the Asian region and for the world.



 Professor Takeshi Iwatsubo
Professor Takeshi Iwatsubo


