Accelerator and brake of microtubule depolymerizing machine Deepening understanding of neuronal circuit formation

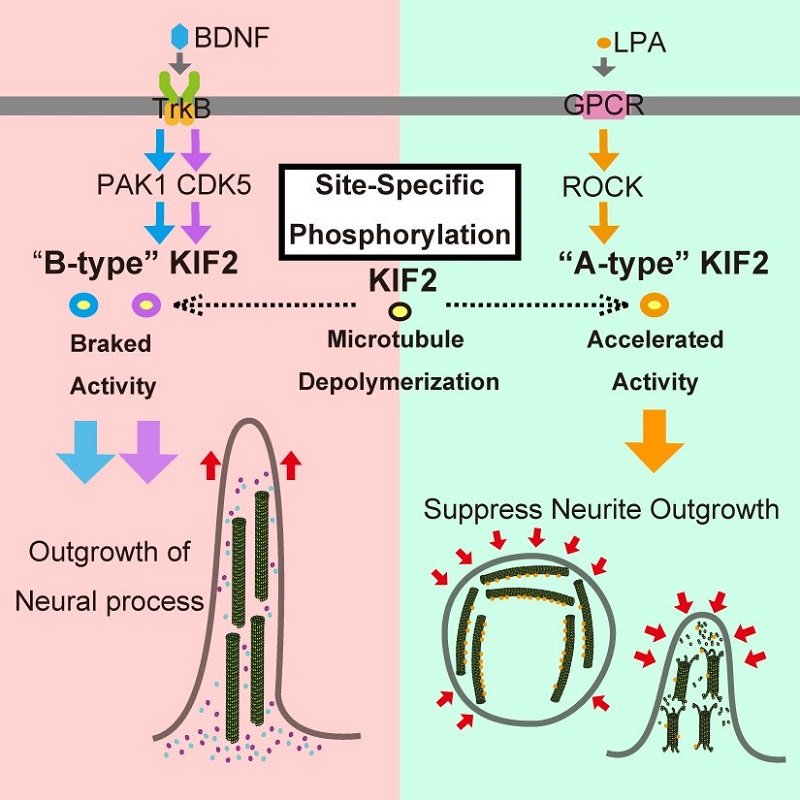
Accelerator and brake of microtubule depolymerizing machine, KIF2
Under BDNF stimulation, which induces growth of neuronal projections, PAK1 and CDK5 kinases phosphorylate specific sites on KIF2, acting as a brake suppressing KIF2’s microtubule depolymerization activity. Under LPA stimulation, which suppresses the growth of neuronal projections, ROCK kinase phosphorylates phosphorylates a different site on KIF2, acting as an accelerator encouraging KIF2’s microtubule depolymerization activity.
© 2015 Hirokawa Lab, The University of Tokyo.
University of Tokyo researchers have revealed a portion of the regulatory mechanism in nerve cells of the growth (polymerization) and contraction (depolymerization) of microtubules that form the skeleton of the cell. This research contributes to our understanding of the mechanisms of brain formation during development.
All cells in our bodies have an internal cytoskeleton formed of microtubules. Microtubules exhibit dynamic structural changes in response to extracellular signals in a dynamic environment or developmental events. Kinesin superfamily protein 2A (KIF2A) has been reported to depolymerize microtubules and to be a part of the regulatory system of the microtubule cytoskeleton in neurons that maintains proper neuronal networks. However, the molecular mechanisms and signaling networks underlying microtubule dynamics remained unknown.
To elucidate the regulatory mechanisms of microtubule dynamics within neurons in response to extracellular signals, Project Professor Nobutaka Hirokawa, Project Assistant Professor Tadayuki Ogawa and their colleagues applied a comprehensive and quantitative analysis combining conventional biological qualitative analysis with systematic quantitative analysis of isolated proteins in test tube conditions, in cultured mammalian cells, and in neurons from the mouse brain. The researchers revealed that different specific kinases phosphorylate KIF2A in different ways, one acting to accelerate (A-type) and the other to brake (B-type) the microtubule depolymerization activity of KIF2A, thus resulting in regulation of the outgrowth of neural processes. The researchers propose that these two mutually exclusive forms of KIF2A phosphorylation differentially regulate neuronal growth in response to extracellular stimuli during development.
Providing a broad and in-depth understanding of the critical molecules for target phenomena can contribute to elucidating the fundamental mechanisms of life and disease onset. “Here, we elucidated the regulatory mechanism of microtubule dynamics in neurons,” says Project Professor Hirokawa. He continues, “This finding will lead to discovering the fundamental mechanism for neuronal circuit formation and contribute to the development of therapeutic drugs for neurodegenerative diseases.”
Press release [PDF] (Japanese)
Paper
, "Microtubule Destabilizer KIF2A Undergoes Distinct Site-specific Phosphorylation Cascades that Differentially Affect Neuronal Morphogenesis", Cell Reports Volume 12, Issue 11, p1774-1788: 2015/9/4 (Japan time), doi: 10.1016/j.celrep.2015.08.018.
Article link (Publication)
Links
Department of Cell Biology and Anatomy, Graduate School of Medicine






