An atomic hole in one!? Enhanced attachment of single platinum atoms on oxygen vacancies in titanium oxide surfaces

Platinum (Pt) is well known as a noble metal which is utilized in many applications ranging from industry to jewelry. Recently, platinum has become a very important material for catalytic applications, particularly for automobiles. However the fundamental mechanism of catalysis reaction and catalyst degradation is still a matter of conjecture, and fundamental understanding of their structures even down to atomic dimensions is needed.
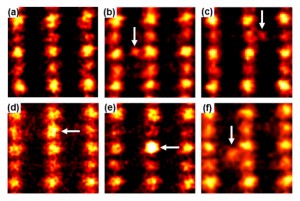
© Naoya Shibata, Atomic-resolution scanning transmission electron micrographs of platinum single atoms attached to titanium oxide surfaces.
(a) No platinum atom, (b)-(f) Platinum atoms attached to the different surfaces sites. The platinum atoms are indicated by the arrows. The present images show that there are at least five different stable attachment sites of platinum atoms on the titanium oxide surfaces.
Researchers at the University of Tokyo Graduate School of Engineering Institute of Engineering Innovation, Associate Professor Naoya Shibata and Professor Yuichi Ikuhara (also at the Japan Fine Ceramics Center and Tohoku University Advanced Institute for Materials Research), in collaboration with Professor Katsuyuki Matsunaga at Nagoya University, have succeeded in the direct observation of platinum single atoms deposited on titanium oxide surfaces and found that the platinum single atoms are preferentially attached to the surface oxygen vacancy sites. The present findings give a fundamental basis for the true understanding of structures, stability and catalysis mechanism of platinum nanoparticles deposited on oxide supports.
The present results have been published in the online edition of Nano Letters published by the American Chemical Society.
Paper
Teng-Yuan Chang, Yusuke Tanaka, Ryo Ishikawa, Kazuaki Toyoura, Katsuyuki Matsunaga, Yuichi Ikuhara and Naoya Shibata,
“Direct imaging of Pt single atoms adsorbed on TiO2 (110)surfaces”,
Nano Letters Online Edition: 2013/12/19, doi: 10.1021/nl403520c.
Article link
Links
Graduate School of Engineering
Institute of Engineering Innovation, Graduate School of Engineering
Crystal Interface Laboratory, Institute of Engineering Innovation, Graduate School of Engineering







