Biosynthesis of natural products with unique structures Biosynthetic machinery for 4-methyloxazoline

The oxazoline ring (C3H5NO) with a methyl group at position 4 is rarely found in natural products; almost all of the methyloxazoline moieties observed in natural products are 5-methyloxazoline. Although 5-methyloxazoline has been shown to be derived from threonine, an amino acid, the means by which the 4-methyloxazoline moiety is produced is unknown.
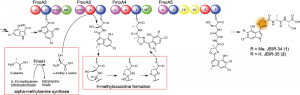
© 2014 Yohei Katsuyama.
Biosynthesis pathway of JBIR-34 and -35. The nonribosomal peptide synthetase FmoA3 utilizes alpha-methylserine, which is synthesized by FmoH, to produce 4-methyloxazoline by heterocyclization. JBIR-34 and -35 are synthesized by the assembly line composed of four nonribosomal peptide synthetases including FmoA3.
Researchers of the University of Tokyo and AIST have been studying the biosynthesis pathway of JBIR-34 and -35 in Streptomyces sp. Sp080513GE-23, which are nonribosomal peptides containing a 4-methyloxazoline moiety. Here, they showed that the biosynthetic origin of the unusual 4-methyloxazoline moiety is alpha-methylserine and identified JBIR-34 and -35 biosynthetic enzymes, including the alpha-methylserine synthase FmoH and the nonribosomal peptide synthetase FmoA3 that utilizes alpha-methylserine to synthesize 4-methyloxazoline by cyclization.
Many nonribosomal peptides, for example vancomycin and daptomycin, have antibiotic properties and are used as pharmaceuticals, and therefore many researchers all over the world aim to produce unnatural nonribosomal peptides by combinatorial biosynthesis, a genetic engineering method. The results from the University of Tokyo and AIST will expand the variety of nonribosomal peptides synthesized by this method and contribute to the development of new drugs.
Paper
Adeline Muliandi, Yohei Katsuyama, Kaoru Sone, Miho Izumikawa, Tomohiro Moriya, Junko Hashimoto, Ikuko Kozone, Motoki Takagi, Kazuo Shin-ya, and Yasuo Ohnishi,
“Biosynthesis of the 4-methyloxazoline-containing nonribosomal peptides, JBIR-34 and -35 in Streptomyces sp. Sp080513GE-23”,
Chemistry and Biology Online Edition: 2014/7/17 (Japan time), doi: 10.1016/j.chembiol.2014.06.004.
Article link
Links
Graduate School of Agricultural and Life Sciences
Department of Biotechnology, Graduate School of Agricultural and Life Sciences攻
Hakko Laboratory, Department of Biotechnology, Graduate School of Agricultural and Life Sciences (Japanese)







