All cations lead to this pathway The structure of channelrhodopsin reveals its cation-conducting pathway and activation mechanism

Researchers at the University of Tokyo’s School of Science have determined the crystal structure of channelrhodopsin (ChR) at atomic resolution, showing its basic architecture and molecular mechanism, and given the long-awaited answer to the question, “Where is the cation conducting pathway?”
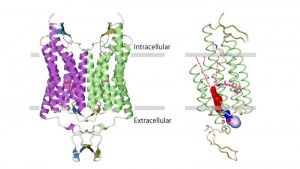
ChR is a light-gated cation channel derived from algae that conducts cations, including sodium ions, in a light-dependent manner. Because the inward flow of sodium ions changes the electrochemical gradient and triggers neuron firing, neurons expressing ChRs can be optically controlled with high temporal precision within systems as complex as freely moving mammals. Although ChR has been broadly applied to neuroscience research, little is known about its molecular mechanism.
Professor Osamu Nureki and graduate student Hideaki Kato at the Graduate School of Science and their collaborators have determined the crystal structure of ChR. The structure revealed the essential molecular architecture of ChR, including the cation conduction pathway. This integration of structural and electrophysiological analyses provides insight into the molecular basis for the remarkable function of ChR, and paves the way for the precise and principled design of ChR variants with novel properties.
Press release (Japanese)
Paper
Hideaki E. Kato, Feng Zhang, Ofer Yizhar, Charu Ramakrishnan, Tomohiro Nishizawa, Kunio Hirata, Jumpei Ito, Yusuke Aita, Tomoya Tsukazaki, Shigehiko Hayashi, Peter Hegemann, Andres D Maturana, Ryuichiro Ishitani, Karl Deisseroth, Osamu Nureki.,
“Crystal structure of channelrhodopsin light-gated cation channel,”
Nature AOP 2012/1/23 (Japan time)
Article link







