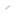Discovery of a new “ice zero” crystalline form of water New explanation for mysterious crystallization behavior of water

Is ice always the same? Until now, only one form of ice was believed to be formed at pressures and temperatures commonly found on Earth. This ice, called Ice I, is the one we commonly experience in our daily lives and that is found everywhere from snowflakes to glaciers. But this belief is about to change, as a new study by researchers at Institute of Industrial Science, the University of Tokyo reveals that water can crystallize also to an entirely new form of ice. The researchers have called this new crystal Ice 0.
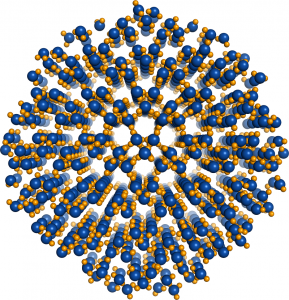
© 2014 John Russo, Flavio Romano, Hajime Tanaka.
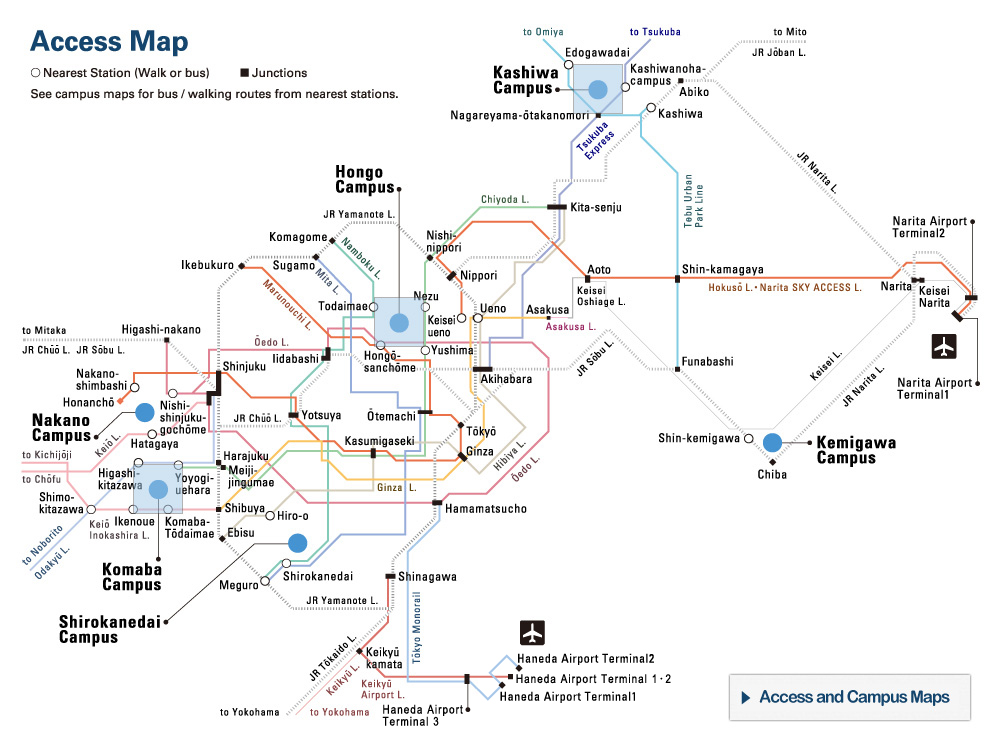
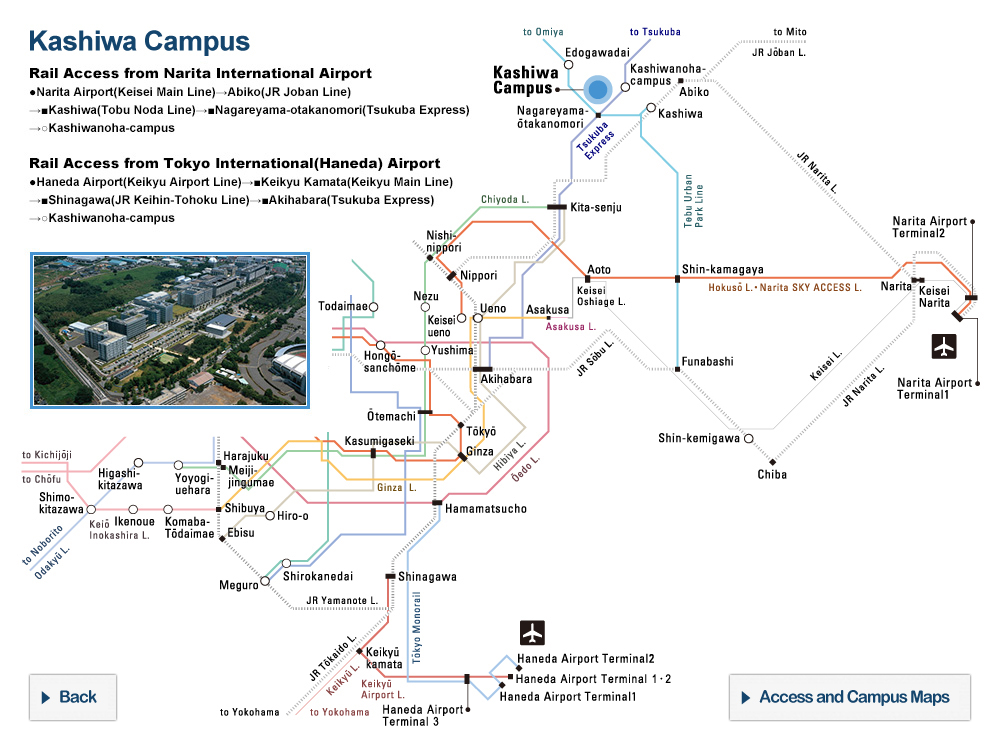
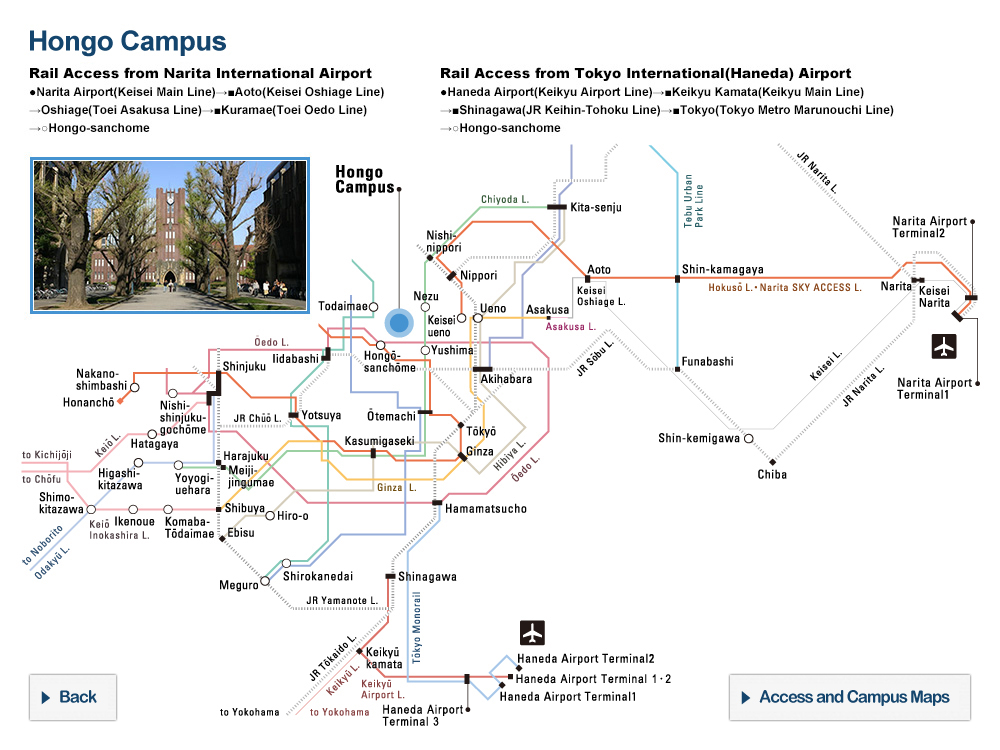
Nucleus of Ice 0. Oxygen atoms are colored in blue, hydrogen atoms in yellow.
The crystallization of water is a fundamental process in many natural environments (e.g. the atmosphere and inside biological systems) and technological settings (e.g. airline, food and energy industries). For example, the ice crystals that form in cirrus clouds play a major role in the Earth’s radiation budget and climate.
Despite its fundamental importance, ice crystallization is still poorly understood. Differently from most liquids, water shows an array of mysterious properties that are also unique in determining the conditions for the appearance and sustainability of life on Earth. One of the striking examples of this behavior is water’s ability to be supercooled without crystallizing. Pure water can in fact be cooled down to -40 degrees without turning into ice, as commonly found in atmospheric clouds, where water droplets can stay liquid despite the low temperatures of the troposphere.
The new research by the research group of Professor Hajime Tanaka and Dr. John Russo at the Institute of Industrial Science, the University of Tokyo has found that at low temperatures water is unable to crystallize in the Ice I form, because the arrangement of the molecules is too dissimilar to what found in the liquid state. This is where the new Ice 0 form comes into play. Thanks to its similarity with the structure of supercooled water, the Ice 0 phase triggers the crystallization process, and then gradually converts into the common Ice I form as the crystals grow in size.
To verify this hypothesis, researchers have performed state-of-the-art numerical simulations and shown that the Ice 0 form correctly explains the mysterious behavior of water under supercooled conditions. This discovery has the potential to radically change our knowledge of ice formation, the most important physical transformation on Earth.
Paper
John Russo, Flavio Romano, and Hajime Tanaka,
“New metastable form of ice and its role in the homogeneous crystallization of water”,
Nature Materials Vol. 13, No. 7, (2014), pp. 733 – 739, doi: 10.1038/nmat3977.
Article link
Links
Institute of Industrial Science
Tanaka Laboratory, Department of Fundamental Engineering, Institute of Industrial Science