Extracting electrons from neutral water using mineral catalyst Proton transfer regulation improves catalytic activity

The research group of Professor Kazuhito Hashimoto and their colleagues at the University of Tokyo Graduate School of Engineering, working with researchers at RIKEN (Team Leader Ryuhei Nakamura and Junior Research Associate Akira Yamaguchi), developed an artificial Manganese (Mn)-based catalyst which can split neutral water to extract electrons by utilizing the same mechanism as the natural photosynthetic system.
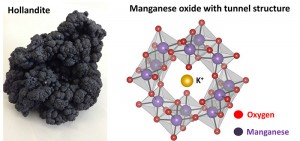
© 2014 Ryuhei Nakamura.
Manganese oxide (MnO2) is the main component of naturally abundant Manganese mineral hollandite (left). Using MnO2 as a catalyst, water oxidation reaction was conducted in the presence of a proton transfer inducer. The MnO2 catalyst possesses a tunnel structure (right).
TWater molecules are one of the most abundant electron sources in nature and are an important chemical resource for creating hydrogen and organic fuels. In nature, photosynthetic organisms such as plants utilize Mn-containing enzymes to obtain electrons from water, using which they then produce carbohydrates from carbon dioxide. Inspired by and mimicking the structure of this enzyme, artificial Mn-based catalysts have been developed to extract electrons from water. Although artificially developed Mn-based catalysts can obtain electrons from water efficiently under acidic or alkaline conditions, their activity drastically decreases under neutral pH. The reason why these catalysts do not work under neutral pH and the origin of the activity difference between the natural and artificial Mn-based catalysts was unknown.
The research group considered the activity difference in terms of the electron and proton transfer mechanism, and investigated the electron and proton transfer process of artificial Mn-based water oxidation catalysts. The research group found that while artificial catalysts transferred the electrons and protons with different timing during the water-splitting process (2H2O → O2 + 4e- + 4H+), natural enzymes transferred them simultaneously. Based on this discovery, the research group intentionally added base reagents which have large proton-accepting ability to the reaction system in order to adjust the timing of the electron and proton transfer. The addition of the base enhanced the water-splitting activity approximately 15 times under neutral pH, and it reached 60 % of activity under alkaline conditions.
This research may lead to the development of means of fuel production with low environmental impact using water, an abundant electron source. The results of this research were published in the scientific journal Nature Communications online edition on June 30, 2014.
Paper
Akira Yamaguchi, Riko Inuzuka, Toshihiro Takashima, Toru Hayashi, Kazuhito Hashimoto and Ryuhei Nakamura,
“Regulating Proton-Coupled Electron Transfer for Efficient Water Splitting by Manganese Oxides at Neutral pH”,
Nature Communications Online Edition: 2014/6/30 (Japan time), doi: 10.1038/ncomms5256.
Article link
Links
Graduate School of Engineering
Department of Applied Chemistry, Graduate School of Engineering
Laboratory of Hashimoto, Department of Applied Chemistry, Graduate School of Engineering
Biofunctional Catalyst Research Team, Center for Sustainable Resource Science, RIKEN







