Materials discovery for earth-abundant battery Battery with new Na-Fe chemistry can surpass lithium-ion

Rechargeable batteries are one of the key technologies required to bring about the future energy saving society, powered by a smart grid and employing electric cars. Lithium (Li) ion batteries offer the most advanced rechargeable energy-storage system, but ever-increasing demand has led to calls for much cheaper alternatives using more common elements. One candidate is the sodium (Na) ion battery, which uses naturally abundant sodium in place of rare lithium. Developing compatible electrode materials has been the subject of intensive research.
© 2014 Atsuo Yamada.
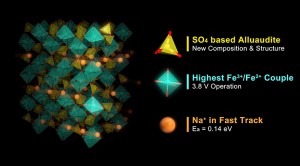
The new material includes a large number of Na+ ions (orange), which can be inserted/extracted along with electrons located around Fe ions (green) during discharge/charge processes. Contrary to the conventional belief that this reaction was impossible in Na-Fe compounds, the present discovery has led to high-energy density system demonstrating high-voltage generation exceeding that of lithium ion batteries and quick charge/discharge in just a few minutes.
Much research to date, however, has focused on screening compounds already used in lithium ion batteries and replacing lithium with sodium, but the poor performance of these materials means they are of limited practical use. Researchers have also tried to combine sodium with other cheap and plentiful elements such as iron (Fe), but experience has shown that creating practical iron-based compounds is extremely difficult.
Professor Atsuo Yamada’s research group at the University of Tokyo’s Graduate School of Engineering has explored Na-Fe based compounds with entirely novel compositions and structures. Now, the group has discovered a new “earth-abundant” cathode material and determined its crystal structure. This new material does not contain any rare metals and can be easily synthesized, enabling the development of sodium-ion batteries with superior performance to lithium-ion batteries. With the novel material as cathode, it is possible to create a battery that generates a high voltage of 3.8 V versus sodium, far exceeding that of current materials. In addition, the extremely fast diffusion of sodium ions can offer quick charge-discharge in just a few minutes.
Paper
Prabeer Barpanda, Gosuke Oyama, Shin-ichi Nishimura, Sai-Cheong Chung, Atsuo Yamada,
“A 3.8 V earth-abundant sodium battery electrode”,
Nature Communications 5:4358 Online Edition: 2014/7/17 (Japan time), doi: 10.1038/ncomms5358.
Article link (publication, UTokyo Repository)
Links
Graduate School of Engineering
Department of Chemical System Engineering, Graduate School of Engineering
Yamada-Okubo Laboratory, Department of Chemical System Engineering, Graduate School of Engineering







