Mechanism for TDP-43 pathology in ALS Calcium-permeable AMPA receptor-mediated calpain activation cleaves TDP-43 into aggregation-prone fragments

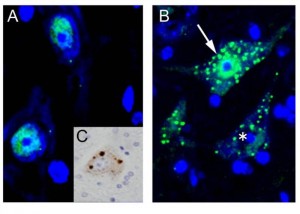
Immunohistochemistry for TDP-43 on the spinal cord of conditional knockout mice for ADAR2 (AR2), an RNA editing enzyme © teamkwak
(A)TDP-43 (green) is localized in the nucleus of motor neurons of normal mice. (B) In the motor neurons of AR2 mice, there are numerous TDP-43-positive inclusions in the cytoplasm (arrow) with reduced or absent (asterisk) TDP-43 immunoreactivity in the nucleus. (C) TDP-43 mislocalization seen in AR2 motor neurons is quite similar to TDP-43 pathology in a spinal motor neuron of an ALS patient (DAB). Green: TDP-43, blue: TO-PRO-3.
Guest Researcher Shin Kwak and Project Researcher Takenari Yamashita at the University of Tokyo’s Graduate School of Medicine, Center for Disease Biology and Integrative Medicine, in collaboration with a RIKEN group, have discovered the molecular mechanism whereby TDP-43 pathology, a pathological hallmark of amyotrophic lateral sclerosis (ALS), occurs in ALS motor neurons. TDP-43 pathology includes absence of TDP-43 from the nucleus and the formation of abnormal cytoplasmic inclusions, and is believed to be involved in the pathogenesis of ALS, but how TPD-43 pathology is formed was poorly understood. This paper demonstrates that the abnormally calcium-permeable AMPA receptor (an ionotropic subtype of the glutamate receptor)-mediated activation of calpain, a calcium-dependent cysteine protease, cleaves TDP-43 into aggregation-prone fragments, which initiates TDP-43 pathology. This finding provides an answer to the unresolved molecular link between the two ALS-specific molecular abnormalities, TDP-43 and down-regulation of ADAR2, an enzyme catalyzing RNA editing (post-transcriptional modification of nucleotides), both of which co-occur in the same ALS motor neurons. Down-regulation of ADAR2 results in the up-regulation of the abnormally calcium-permeable AMPA receptor by failure of naturally occurring RNA editing of GluA2, a subunit of the AMPA receptor. Furthermore, expression of unedited GluA2 is a cause of death of motor neurons in conditional ADAR2 knockout mice (AR2), a mechanistic model of sporadic ALS. The researchers found that activated calpain specifically mediates TDP-43 pathology in AR2 mice. Additional demonstration of calpain-dependent TDP-43 fragments in the spinal cord and brain of ALS patients, and high vulnerability of ALS-linked mutant TDP-43 to cleavage by calpain support the crucial role of the calpain-dependent cleavage of TDP-43 in the ALS pathology.
Press release [PDF] (Japanese)
Paper
Masahiro Yuki, Hiromasa Tanaka, Kouitsu Sasaki, Yoshihiro Miyake, Kazunari Yoshizawa, Yoshiaki Nishibayashi,
“Iron-Catalyzed Transformation of Molecular Dinitrogen into Silylamine under Ambient Conditions”,
Nature Communications Online Edition: 2012/12/5 (Japan time), doi: 10.1038/ncomms2264.
Article link
Links
Center for Disease Biology and Integrative Medicine,Division of Clinical Biotechnology
Labo teamkwak (Japanese)







