Mechanism of biological multi-fuel engine Elucidating flagellar motor ion transfer process

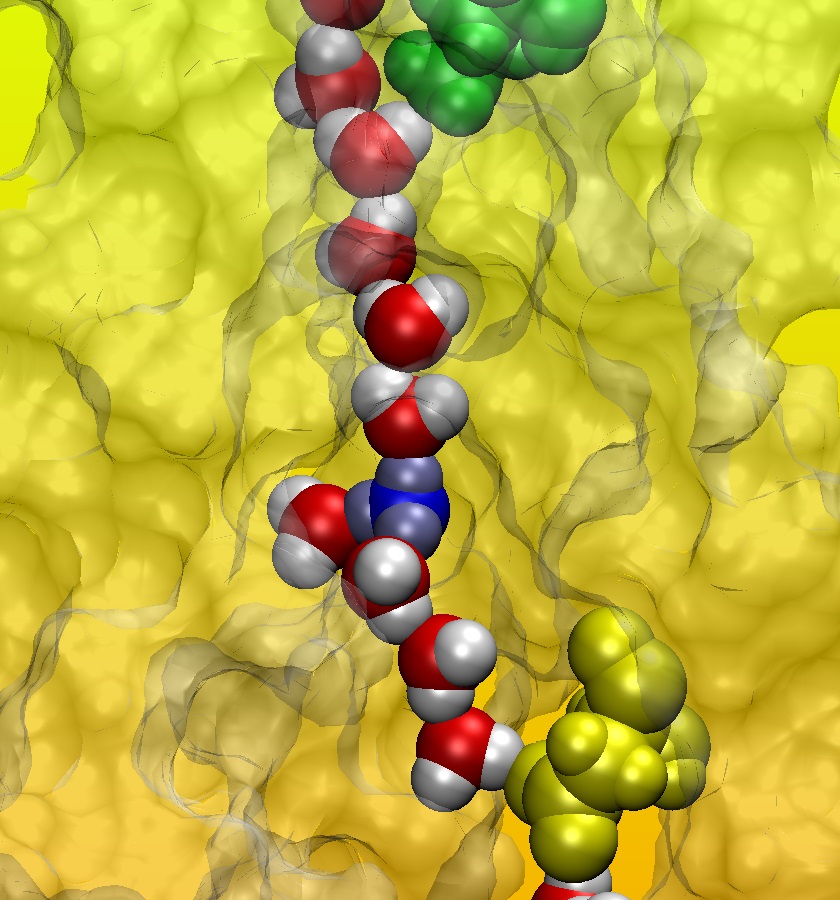
Proton permeation through flagellar motor stator complex MotA/B
Based on the model of the three-dimensional structure of MotA/B identified in this research, protons can permeate through the gate (green) of the motor by diffusion of hydronium ions (blue), which induces the formation of a water wire (red and white) that may mediate the proton transfer to the proton binding site (yellow).
© 2015 Yasutaka Nishihara and Akio Kitao.
University of Tokyo researchers have constructed the atomic model structure of the protein complex that corresponds to the stator (stationary part of a motor that surrounds the rotating part of a motor) of the E. coli flagellar motor for the first time by molecular simulation based on previously published experimental data, and elucidated the mechanism by which ions, including hydrogen ions (protons), are transferred through the stator.
Bacteria such as E. coli and Salmonella swim by rotating flagellar motors and filaments, which highly efficiently utilize the energy originating from the difference in ion concentration between the cell interior and exterior. Among the bacterial flagellar motors, some convert the energy by the permeation of protons through the motor stator, while others utilize sodium ions or multiple ions. However, the atomic structure of the bacterial flagellar motor remained unknown, and the mechanism of ion permeation had not been elucidated in detail.
Project Researcher Nishihara Yasutaka at the Graduate School of Arts and Sciences and Associate Professor Akio Kitao at the Institute of Molecular and Cellular Biosciences constructed a three-dimensional model structure of the protein complex that comprises the flagellar motor stator MotA/B, and found that protons permeate through the transmembrane stator as hydronium ions, inducing a motion similar to a ratchet wrench (ratchet movement) limited to one directional rotation.
Investigation of this type of highly efficient energy conversion mechanism is essential to understand biological mechanisms which can utilize energy efficiently.
Press release (Japanese)
Paper
, "Gate-controlled proton diffusion and protonation-induced ratchet motion in the stator of the bacterial flagellar motor", Proceedings of the National Academy of Science of the United States of America Online Edition: 2015/6/9 (Japan time), doi: 10.1073/pnas.1502991112.
Article link (Publication)
Links
Institute of Molecular and Cellular Biosciences
Laboratory of Computational Protein Science, Institute of Molecular and Cellular Biosciences






