Mechanism of caveolae-mediated endocytosis of LRP1 KIF13B, a go-between for LRP1 and caveolae

Endocytosis, the process by which cells uptake external material, is important for the elimination of the invading bacteria from the body, the removal of LDL and HDL (low/high density lipoprotein; transporters of cholesterol) from the blood stream, and so on. Endocytosis occurs by two pathways, clathrin-dependent (via vesicles coated with the protein clathrin) and calveolin-dependent (via caveolae, small invaginations rich in the protein caveolin on the cell surface). While clathrin-dependent endocytosis has been well studied, little is known about caveolin-dependent endocytosis.
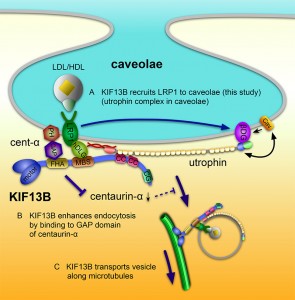
© Kanai Y. et al., 2014. Originally published in The Journal Cell Biology. doi: 10.1083/jcb.201309066.
Proposed model for the role of KIF13B in caveolae-dependent endocytosis of LRP1. (A) KIF13B links LRP1 to caveolae through the LRP1-hDLG1-KIF13B-utrophin-caveolae linkage (this study). (B) KIF13B activates Arf6 through the inhibition of Arf6-GAP activity of centaurin-α1, subsequently enhancing the endocytosis nearby (Venkateswarlu et al., 2005). (C) KIF13B, as a motor protein, transports the endocytosed vesicles along microtubules (Hirokawa et al., 2010).
The research group of Professor Nobutaka Hirokawa and Associate Professor Yoshimitsu Kanai at the University of Tokyo Graduate School of Medicine has established that a microtubule-based motor protein, KIF13B, in an unexpected and unconventional function, enhances caveolin-dependent endocytosis of LRP1 (lipoprotein receptor related protein-1) by linking LRP1 and caveolae. LRP1 is a membrane receptor protein involved in the uptake of LDL and HDL into the cell. KIF13B was abundant in the liver and was localized on the sinusoidal plasma membrane of hepatocytes, where the cells are directly facing to the blood stream in the liver. KIF13B knockout (KO) mice that could not express KIF13B protein showed elevated levels of serum cholesterol, and KO mouse embryonic fibroblasts showed decreased uptake of LDL. Further, KIF13B bound to hDLG1 and utrophin, which, in turn, bound to LRP1 and caveolae, respectively. These linkages were required for the KIF13B-enhanced endocytosis of LRP1. Therefore, the group has proposed that KIF13B, working as a scaffold, recruits LRP1 to caveolae via LRP1-hDLG1-KIF13B-utrophin-caveolae linkage and enhances the endocytosis of LRP1 and promotes the uptake of blood cholesterol into cells.
These results shed light on the mechanism of the caveolae-mediated endocytosis, and provide a new therapeutic approach for caveolae-related diseases such as hypercholesterolemia and cancers.
Paper
Y. Kanai, D. Wang, N. Hirokawa,
“KIF13B enhances the endocytosis of LRP1 by recruiting LRP1 to caveolae”,
Journal of Cell Biology, vol. 204, no. 3, p. 395-408, doi: 10.1083/jcb.20130966.
Article link
Links
Molecular Cell Biology, Department Cell Biology and Anatomy, Graduate School of Medicine






