New carboxylic acid selective carbon-carbon bond forming reactions Synthesis of amino acids and drug candidates from acetic acid

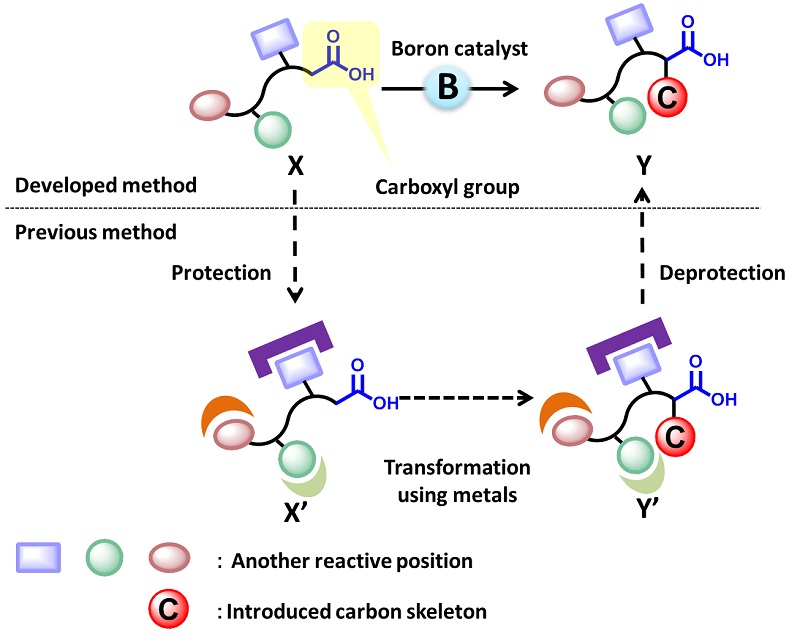
The newly developed method and previously known method
When transforming X to Y, it was necessary to go through extra steps at X’ and Y’ to“protect” undesired positions on the molecule (below). In contrast, the new method enables direct transformation from X to Y by a boron catalyst (above).
© 2015 Motomu Kanai.
A University of Tokyo research group has successfully developed a novel method enabling selective transformation of carboxyl groups, a common structure in many drugs and natural products.
Direct transformation of biologically relevant molecules with complex structures has the potential to modify the biological activity and other properties of these compounds. However, it has been very difficult to promote the desired transformation at the desired position of the molecule.
The research group of Professor Motomu Kanai and Assistant Professor Yohei Shimizu in the Graduate School of Pharmaceutical Sciences has successfully installed new carbon skeletons at the carboxyl group of various molecules by employing a boron catalyst which has high affinity to an oxygen atom of the carboxyl groups. The developed reaction was able to transform anti-inflammatory drugs, such as loxoprofen or indomethacin, and natural products, such as the plant hormone jasmonic acid.
Previously known methods for similar transformations required large amounts of metals and harsh conditions making it difficult to control the reaction site and producing undesired side reactions. As a result it was difficult to apply such methods to drugs and other molecules with complex structures. Therefore, “protection” of undesired reaction sites by converting them into unreactive structures was necessary to prevent side reactions under the previous methods. In contrast, the newly developed method does not require protection due to selective activation of carboxyl group by a tiny amount of the boron compound catalyst and can take place under mild conditions without requiring the presence of metals.
The same boron catalyst enabled the synthesis of variety of β-amino acids from a very simple carboxylic acid, acetic acid, the main component of vinegar. Compared to previous β-amino acid synthesis methods, this method decreases the required number of synthetic steps and byproducts, potentially leading to cost effective and environmentally friendly synthesis of β-amino acids.
Since the carboxyl group is an extremely common structure in drugs and drug candidate compounds, a method enabling selective transformation of carboxyl groups will be a powerful tool in developing new drugs.
Paper
, "Chemoselective Boron-Catalyzed Nucleophilic Activation of Carboxylic Acids for Mannich-Type Reactions", Journal of the American Chemical Society Online Edition: 2015/05/27 (Japan time), doi: 10.1021/jacs.5b04175.
Article link (Publication)






