New therapeutic strategy for Alzheimer’s disease by catalysis Photooxygenation catalysts that sense amyloid-β structures

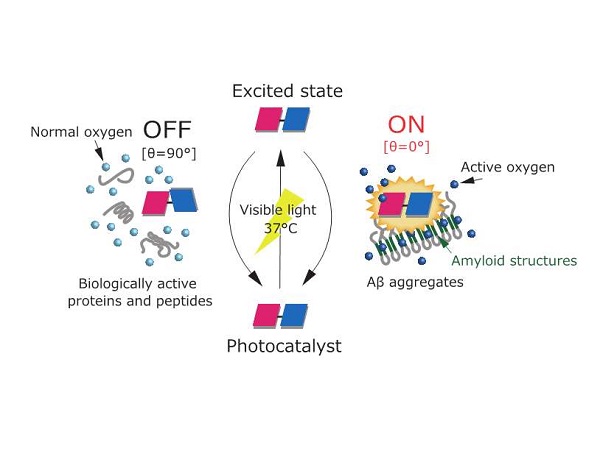
Oxygenation mechanism of the photocatalyst Mechanism of differentiating Aβ aggregates The photocatalyst is excited into a high energy state by irradiation with visible light. When it returns to its original state it binds to amyloid structures (shown in green) of Aβ aggregates. Oxygen is generated and Aβ aggregates are oxygenated only when the angle θ of the red and blue faces of the photocatalyst are fixed near zero. On the other hand, the photocatalyst cannot oxygenate biologically active peptides or proteins that do not contain amyloid structures.
© 2016 Motomu Kanai.
A University of Tokyo research group has developed a new photocatalyst that can sense and selectively oxygenate only amyloid structures of aggregates of amyloid-β (Aβ), a peptide involved in the onset of Alzheimer's disease, and suppress their aggregation. The group showed that aggregation activity and toxicity were remarkably suppressed in the oxygenated Aβ aggregates.
Damage to nerve cells by aggregates of Aβ is thought to be involved in the onset of Alzheimer's disease. As a result, researchers have sought therapies to suppress the aggregation of Aβ. The group has been seeking to establish new Alzheimer’s treatments using catalysis, and previously developed a photocatalyst that suppressed the aggregation of Aβ by oxygenating Aβ when irradiated with light. However, in addition to Aβ, the catalyst also oxygenated other biomolecules that play important roles in the body at the same time.
A highly selective photocatalyst that oxygenated only when bound to Aβ was needed in order not to oxygenate other biomolecules, so the research group developed a photocatalyst (a low molecular weight organic compound) that distinguished a specific amyloid structure only found in Aβ aggregates. The group found that oxygenation of Aβ in a test tube with the photocatalyst suppressed the further aggregation of Aβ. Further, when the photocatalyst was coupled with a peptide that recognizes Aβ, the photocatalyst distinguished amyloid structures and oxygenation of Aβ proceeded in cells in the test tube, reducing the toxicity of Aβ aggregates to cells.
In the future, enabling this catalyst to oxygenate a substrate even with the application of a tiny amount of light energy and making further improvements to minimize impact on the body, may lead to new treatments for Alzheimer's disease using catalytic reactions.
Sohma, who managed the research in the laboratory, says, “We had to modify the catalysts many times by trial and error, but finally we successfully developed a catalyst that can sense and selectively oxygenate only amyloid structures of Aβ aggregates.” He continues, “Next we will try to apply our catalyst to in vivo animal experiments.”
The results of this research were published in the online edition of the British scientific journal Nature Chemistry on 27 June 2016 (UK time).
Press release (Japanese)
Paper
, "Switchable Photooxygenation Catalysts that Sense Higher-Order Amyloid Structures", Nature Chemistry Online Edition: 2016/06/28 (Japan time), doi: 10.1038/nchem.2550.
Article link (Publication)
Links
Graduate School of Pharmaceutical Sciences







