Novel gene turns cardiac muscle into smooth muscle Learning from fish heart evolution

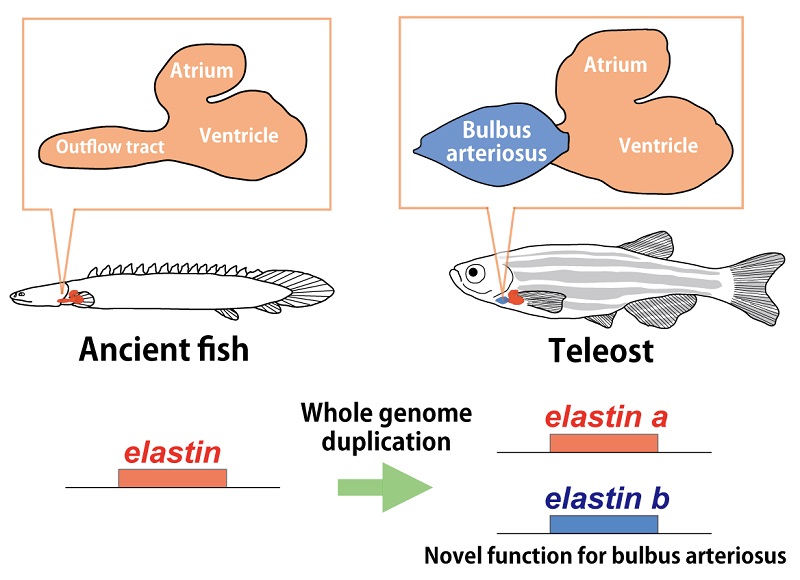
The mechanism underlying teleost heart evolution
Whole genome duplication occurred in the course of teleost evolution and an elastin gene was duplicated to elastin a and elastin b. Mutations were accumulated in elastin b, which acquired a new function for bulbus arteriosus development.
© 2016 Yuuta Moriyama.
Researchers at the University of Tokyo have discovered the novel gene elastin b which was acquired in the course of fish evolution and regulates cell fate determination, turning cardiac muscle into smooth muscle. In this way fish may have specialized their heart and adapted to an aquatic environment.
The heart is the first organ to form and function during development. The main function of heart is the circulation of oxygenated blood to the entire body, so the heart is an organ that can be regarded as central to “life” itself. Animals have evolved and specialized their hearts to adapt to their living environments, resulting in tremendous variation in heart shape and function. In teleosts, the vertebrate that has flourished most successfully, the heart outflow tract is transformed into a novel organ called the “bulbus arteriosus” by changing cell types from cardiac muscle into smooth muscle in the process of their evolution and development. The bulbus arteriosus is regarded as one of the most important teleost organs for their adaptation to an aquatic environment and subsequent explosive diversification and spread. However, the mechanisms underlying the development and evolution of the bulbus arteriosus are largely unknown.
Here, the research group of Dr. Yuuta Moriyama and Lecturer Kazuko Koshiba-Takeuchi and their colleagues at the University of Tokyo, Institute of Molecular and Cellular Biosciences has revealed that the teleost-specific extracellular matrix gene, elastin b, was acquired in the course of teleost evolution and that regulates cell fate, causing cardiac muscle cells to develop instead into smooth muscle and thereby contributing to the development of the bulbus arteriosus. Elastin b is known to encode for the extracellular matrix, the filling of extracellular space. This is the first report elucidating the emergence of a novel organ by alteration of the extracellular environment in animal evolution.
“These findings offer new understanding about a fundamental question in the life sciences – how organisms acquire new organs in the process of evolution,” says Dr. Moriyama. He continues, “At the same time, this research may contribute to medical and regenerative studies of artificial modification of cardiac cell differentiation.”
Press release (Japanese)
Paper
, "Evolution of the fish heart by sub/neofunctionalization of an elastin gene", Nature Communications Online Edition: 2016/1/19 (Japan time), doi: 10.1038/ncomms10397.
Article link (Publication)
Links
Institute of Molecular and Cellular Biosciences
Division of Cardiovascular Regeneration, Institute of Molecular and Cellular Biosciences (Japanese)






