One step toward the dream of surgical nanomachines Biomolecular machine releases drugs upon detecting ATP

Realization of “surgical nanomachines”, proposed by physicist Richard Feynman, is undoubtedly one of the ultimate scientific challenges. Such nanomachines are expected to detect particular biological (endogenous) signals and judge the necessary tasks to cure wounded tissues.
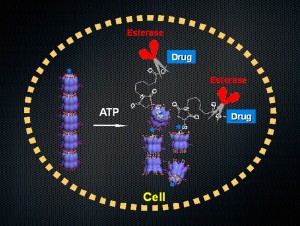
© Takuzo Aida. The nanocarrier, formed by the assembly of biological machine GroEL, when taken up into cells breaks up upon binding with intracellular ATP and exposes a drug-appended guest protein to the intracellular environment containing enzymes, which then cleave off a linker to liberate drugs from the protein scaffold.
Inspired by this vision, the Aida research group at the University of Tokyo’s Graduate School of Engineering has developed the first robotic nanocarrier that senses ATP (adenosine-5’-triphosphate) and breaks up spontaneously to release a guest molecule. This unprecedented nanocarrier consists of tubularly assembled GroEL, a barrel-shaped chaperonin protein, ubiquitously present in the human body as an essential biomolecular machine that assists refolding of denatured proteins by trapping them inside its barrel. For GroEL to release refolded proteins from the cavity, it binds ATP which is then hydrolyzed to create ADP, wherein GroEL undergoes a large conformational change. The researchers took note of this ATP-fueled chemomechanical motion and hypothesized that if the mechanical force generated by this motion is large enough to cut non-covalent interactions, the tubular GroEL assembly may break up spontaneously into its monomer upon binding with ATP and release a guest molecule. This idea was realized in this research.
The nanocarrier has the potential to differentiate biological environments in terms of ATP concentration and release guests in ATP-rich regions. The concentration of intracellular ATP is in a range of 1?10 mM, while that of extracellular ATP is very low (~5 μM), except around inflammatory tissues such as tumor tissues, where extracellular ATP concentrations are high at around 1 mM. The researchers found that nanocarrier scission occurs with a sigmoidal dependency on ATP concentration. Accordingly, the scission occurs inside cells but not in serum.
For the realization of drug delivery with the GroEL-based nanocarrier, one can utilize a denatured alpha-lactalbumin as a protein guest for anchoring multiple drugs via an enzymatically cleavable linker. When the nanocarrier is taken up into cells and breaks up by the action of intracellular ATP, this drug-appended lactalbumin guest is exposed to an intracellular environment containing an enzyme that can cleave off the linker to liberate drug molecules from the lactalbumin scaffold. Although the nanocarrier developed for this paper was not designed for long-term blood circulation, a preliminary biodistribution test using tumor-carrying mice revealed that it is preferentially accumulated in a tumor tissue over other tissues except for the liver. This work opens a new avenue for drug delivery systems (DDS) using biomolecular machines as carriers.
Paper
Shuvendu Biswas, Kazushi Kinbara, Tatsuya Niwa, Hideki Taguchi, Noriyuki Ishii, Sumiyo Watanabe, Kanjiro Miyata, Kazunori Kataoka, Takuzo Aida,
“Biomolecular robotics for chemomechanically driven guest delivery fuelled by intracellular ATP”,
Nature Chemistry Online Edition: 2013/6/2 (Japan time), doi: 10.1038/nchem.1681.
Article link
Links
Graduate School of Engineering
Department of Chemistry and Biotechnology, Graduate School of Engineering






