Possible ErbB-NF-κB pathways in breast cancer stem cells for tumorigenesis

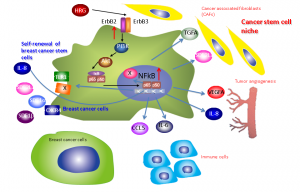
HRG-ErbB-PI3 kinase-NFκB axis may regulate self-renewing breast cancer stem cells and induce production of extracellular proteins for generating cancer stem cell niche. © Noriko Gotoh.
Researchers at the University of Tokyo Institute of Medical Science and Graduate School of Medicine have discovered mechanisms that may be essential for the maintenance of cancer stem cells. Cancer stem cells (CSCs), which make up only a small proportion of heterogeneous tumor cells, may possess a greater ability to maintain tumorigenesis than do other tumor cell types. CSCs can self-renew and simultaneously produce differentiated daughter cells; thus they can strongly proliferate until they reach their final differentiated state. Because most cancer cells proliferate extensively, traditional cancer therapies aim to eliminate as many cancer cells as possible by targeting cells with increased proliferation activity. However, relapse occurs in a significant number of patients even after complete tumor resection and systemic treatment involving chemotherapy and/or radiotherapy. This is because CSCs are relatively resistant to conventional chemotherapy and radiotherapy and might survive after systemic treatment. These cells may remain dormant for years but eventually cause relapse. Therefore, cancer therapy should target CSCs that were not targeted by conventional therapy. Although the concept of CSCs greatly influences cancer biology and evokes reconsideration of cancer treatment, the molecular mechanisms underlying the contribution of these cells to tumorigenesis remain obscure. To develop more effective cancer therapies, it is critical to understand the mechanism by which CSCs are maintained in the body.
The researchers have discovered possible molecular mechanisms that are critical for maintenance of self-renewing breast CSCs in the body. They showed that Heregulin (HRG), a ligand for ErbB3 receptor tyrosine kinase, is able to maintain mammospheres, in vitro-cultured cell aggregation, that are derived from single cancer stem-like cells in the breast cancer tissues of the patients. These findings further demonstrate that ErbB-phosphatidyl inositol 3-kinase (PI3K)-NF-κB axis is essential for the HRG-induced mammosphere formation. The HRG-induced activation of NF-κB induces production of proinflammatory cytokines and chemokines, including interleukin-8 (IL-8) for self-renewal of breast CSCs, vascular epidermal growth factor (VEGF) for angiogenesis and CCL5 for immune response. These paracrine factors may actively generate and maintain a microenvironment for the survival and proliferation of breast CSCs, or in other words, a breast CSC niche. It is thus important to target molecules involved in the ErbB-PI3K-NF-κB pathway in order to eradicate breast cancer.
Paper
Kunihiko Hinohara, Seiichiro Kobayashi, Hajime Kanauchi, Seiichiro Shimizu, Kotoe Nishioka, Ei-ichi Tsuji, Kei-ichiro Tada, Kazuo Umezawa,Masaki Mori, Toshihisa Ogawa, Jun-ichiro Inoue, Arinobu Tojo, and Noriko Goto,
“ErbB receptor tyrosine kinase/NF-κB signaling controls mammosphere formation in human breast cancer”,
PNAS April 5, 2012 doi: 10.1073/pnas.1113271109
Article link
Links
Division of Systems Biomedical Technology
Division of Cellular and Molecular Biology (Japanese)







