Quick-charging lithium-ion batteries on the horizon Unusual properties of superconcentrated electrolyte

Secondary (rechargeable) batteries that can store and release electricity at need are a core technology and key to realizing an energy-efficient society based on use of smart grids and electric vehicles. Currently the lithium-ion battery is the most advanced secondary battery technology, and considerable effort has been devoted to the development of advanced lithium-ion batteries with high-voltage and fast-charging characteristics. However, among the components of lithium-ion batteries in particular, there has been little advance in electrolyte materials during the past two decades, and development of an innovative electrolyte may be the key to the next great breakthrough in advanced lithium-ion batteries.
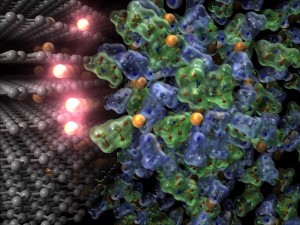
© Yuki Yamada.
Charging reaction of lithium-ion batteries. Lithium ions (orange spheres) move from the electrolyte (right) to the anode (left). Ethylene carbonate solvent was required as electrolyte for this reaction, but the current research has made it possible to use a great variety of superconcentrated electrolytes as solvent. The new electrolyte developed in this research remarkably increases the reaction kinetics and thus contributes to the development of fast-charging lithium-ion batteries that can be charged in just one-third of the time of conventional batteries.
Assistant Professor Yuki Yamada and Professor Atsuo Yamada at the University of Tokyo, Graduate School of Engineering, Department of Chemical System Engineering, Dr. Keitaro Sodeyama at Kyoto University, and Dr. Yoshitaka Tateyama at the National Institute of Materials Science have developed a new electrolyte for fast-charging and high-voltage lithium-ion batteries. The new electrolyte is an organic solution containing a superhigh concentration of lithium ions, over four times more concentrated than current electrolytes. The group has discovered unusual stability and fast reaction kinetics in the superconcentrated electrolyte, far exceeding currently used commercial electrolytes. The group have revealed that the origin of these peculiar properties is due to a particular solution structure by molecular dynamics simulations using the K supercomputer at RIKEN.
The new electrolyte will open up the way to development of advanced lithium-ion batteries that can be charged in a third of the time of conventional lithium-ion batteries and deliver voltages much greater than the current limit of 3.7 V and as high as 5 V, which will enable their use in applications requiring high energy densities such as electric vehicles and smart grids.
Paper
Yuki Yamada, Keizo Furukawa, Keitaro Sodeyama, Keisuke Kikuchi, Makoto Yaegashi, Yoshitaka Tateyama, Atsuo Yamada,
“Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries”,
Journal of the American Chemical Society Online Edition: 2014/3/24, doi: 10.1021/ja412807w.
Article link
Links
Graduate School of Engineering
Department of Chemical System Engineering, Graduate School of Engineering
Yamada Lab, Department of Chemical System Engineering, Graduate School of Engineering







