Structure of an intracellular sensor in innate immune system An inactive closed conformation of NOD2 mediated by ADP

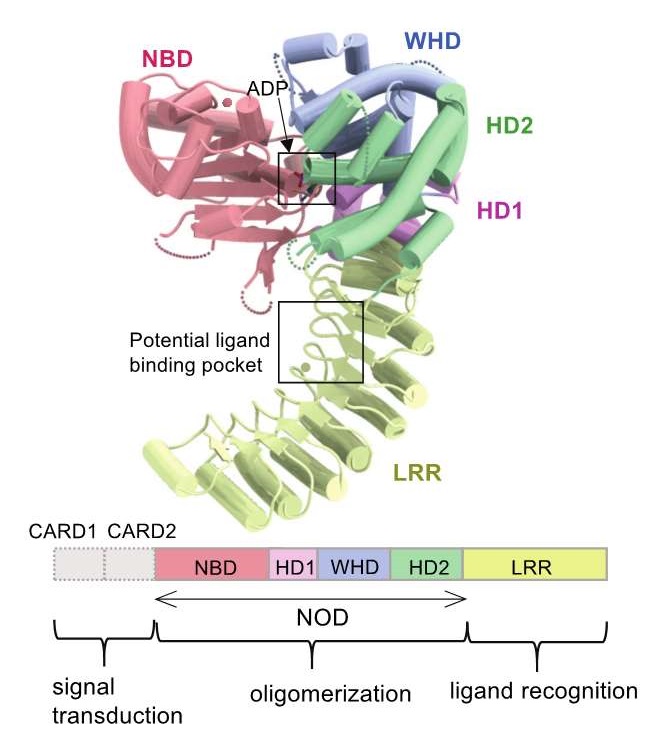
Overall structure of NOD2
NOD2 consists of a several subdomains, which are displayed in different colors. The lower panel is a schematic representation of the domain organization of NOD2.
© 2016 Toshiyuki Shimizu
Researchers at the University of Tokyo have demonstrated the structure of a NOD receptor, an intracellular sensor that detects invasion of pathogens as part of the innate immune system. This research is an important step towards elucidating the mechanism by which NODs are activated and the processes by which the immune system detects pathogens.
Our bodies are equipped with an innate immune system that includes sensor proteins within our cells that detect infection by pathogens such as bacteria and viruses. One such group of sensor proteins is the NOD-like receptors (NLRs), which detect what is called a nucleotide-binding oligomerization domain (NOD). This group of proteins is activated by bonding with its target ligand and then forming a large multiprotein complex, and is known to transmit information of the pathogen infection within the cell. However, some aspects of the three-dimensional structure remain unclear and it was thought that clarifying the three-dimensional structure would deepen our understanding of the role of NLRs.
The research group of Professor Toshiyuki Shimizu at the Graduate School of Pharmaceutical Sciences used X-ray crystallography to reveal the three-dimensional structure of NOD2, an NLR, while bound to ADP, the energy source of cells, and also its structure in an inactive state. As a result, the research group found that ADP mediates the interaction of domains with different functions within the NOD2 protein, and discovered a pocket-like structure in a leucine-rich repeat (LRR) domain that may be involved in ligand recognition.
There are many reports of NOD2 proteins with just single nucleotide differences called single nucleotide polymorphisms (SNPs), and it is known that NOD2 single nucleotide polymorphisms are associated with autoinflammatory diseases such Blau syndrome and Crohn's disease. For this reason, the research group, based on the three-dimensional structure discovered in this research, also discussed the relationship between SNPs and these diseases.
“Among the sensors in innate immune system, an understanding of the structure of NLR remains largely unknown. This study is a big step towards understanding the regulation mechanism of NLR,” says Shimizu. He continues, “The active form of NOD2 should be revealed by further studies.”
Paper
, "Crystal structure of NOD2 and its implications in human disease", Nature Communications: 2016/6/10 (Japan time), doi: 10.1038/ncomms11813.
Article link (Publication)
Links
Graduate School of Pharmaceutical Sciences
Laboratory of Protein Structural Biology, Graduate School of Pharmaceutical Sciences







