Study reveals molecular mechanism of dual-function motor protein KIF19A protein’s structure allows it to carry out double roles

Structure and dual functions of KIF19A motor domain
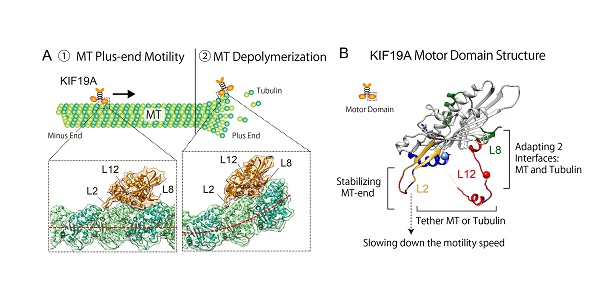
(A) KIF19A moves along the microtubule (MT) and depolymerizes the microtubule when it reaches the plus end. The flexible binding surface of KIF19A adapts to both the microtubule and tubulin (microtubule-forming protein) interface.
(B) X-ray crystal structure and functional anatomy of KIF19A motor domain
© 2016 Nobutaka Hirokawa
A University of Tokyo research group has unveiled the three-dimensional crystal structure of a molecular motor protein called KIF19A and revealed the mechanism by which it controls the length of cilia, the hair-like structures of cells lining the body’s passages, at the atomic level.
Kinesin superfamily proteins (KIFs) are found in a wide variety of organisms, from budding yeast to humans. Some KIFs move fluids along the microtubule, a small tube that serves as an intercellular track, while other types of kinesin motor proteins break down this microscopic tubular structure. KIF19A, a type of KIF motor protein found in the cilia of oviducts (such as the fallopian tubes), ventricles (cativities of the brain containing fluid), and trachea, travels toward the tip, or plus end, of microtubules and disassembles (depolymerizes) them, thereby acting to adjust the length of cilia. KIF19A is unique among KIFs in that it functions as both courier and demolition expert; however, scientists knew very little about the molecular mechanisms of KIF19A’s dual functions.
The research group led by Project Professor Nobutaka Hirokawa at the University of Tokyo’s Graduate School of Medicine was able to show in detail the three-dimensional structures of KIF19A and the protein bound to the microtubule by employing the imaging and investigative methods of X-ray crystallography and cryo-electron microscopy. By creating a mutant KIF19A in the lab, based on the information they gained on the structural properties of the motor protein, and analyzing its biochemical and biophysical functions, the researchers identified structural elements L2, L8, and L12 as playing key roles in allowing the protein to carry out its dual functions. The findings show that these three structural elements work within KIF19A to enable motility and microtubule depolymerization at the microtubule’s tip.
The current study may eventually lead to genetic diagnosis and therapy for hydrocephalus (a disorder characterized by accumulation of fluid in the brain), female infertility, and other diseases related to abnormal cilia length caused by defective KIF19A.
“We were able to illustrate the interaction between the dual-function KIF19A protein and the microtubule with both structural and functional analyses,” says Hirokawa. He continues, “Genetically altered mice with inactivated KIF19A developed abnormally long cilia, displaying traits of hydrocephalus and female infertility similar to human diseases. I am hopeful that the findings from our study will contribute to our understanding of these diseases.”
Paper
, "Motility and Microtubule Depolymerization Mechanisms of the Kinesin-8 motor, KIF19A", eLife Online Edition: 2016/09/30 (Japan time), doi: 10.7554/eLife.18101.
Article link (Publication)
Links
Department of Molecular Cell Biology, Graduate School of Medicine (Japanese)







