Synthetic virtuosity of a bacterial enzyme Enzyme assembles complex carbon skeleton in one go

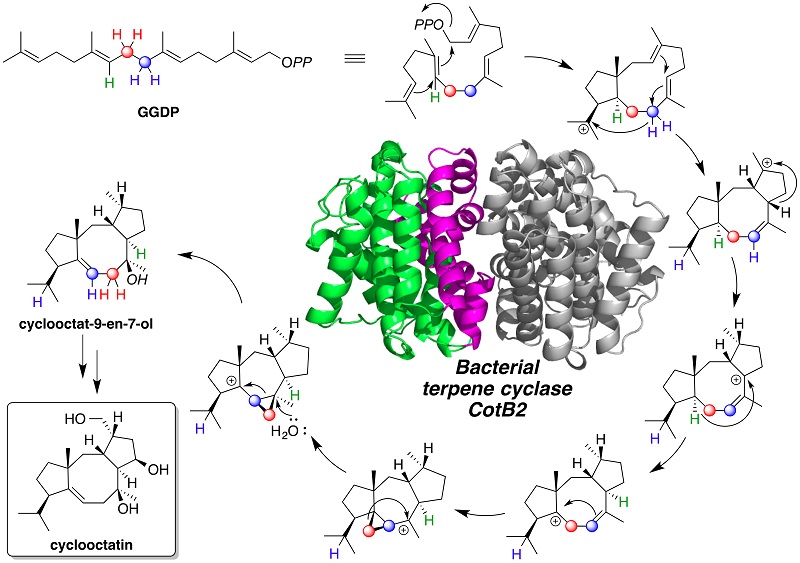
Terpene compounds include important natural products for medicines and fragrances. Cyclooctatin, which is also a terpene compound, is produced by an actinomycete Streptomyces melanosporofaciens MI614-43F2 and shows anti-inflammatory activity. The carbon skeleton of cyclooctatin is assembled by the action of terpene cyclase CotB2, which catalyzes the cyclization of geranylgeranyl diphosphate (GGDP) to produce cyclooctat-9-en-7-ol. A plausible mechanism has been previously proposed for the CotB2-catalyzed cyclization reaction, where the characteristic 5-8-5 fused ring system of cyclooctat-9-en-7-ol having six chiral centers is synthesized by cyclization of the C20 achiral GGDP. However, no direct experimental evidence has been provided to validate the proposed mechanism for CotB2-catalyzed cyclization reaction.
Researchers at Biotechnology Research Center (BRC) of the University of Tokyo, Osaka City University, and the Institute of Microbial Chemistry, Tokyo, proposed a new mechanism for the exquisite CotB2-catalyzed cyclization, by investigating the CotB2-catalyzed reaction in great detail. This study demonstrated that the mechanism for the CotB2-catalyzed reaction involves dissociation of the pyrophosphate leaving group of GGDP, an unusual carbon backbone rearrangement, and a series of long-range hydride shifts (figure). Thus, CotB2 elegantly demonstrates the biosynthetic virtuosity that evolution has conferred on terpene synthases.
This result may lead to the development of novel biocatalysts useful for the production of a diverse range of complex compounds that exhibit biological activity.
Paper
, "An Unusual Terpene Cyclization Mechanism Involving a Carbon-Carbon Bond Rearrangement", Angewandte Chemie International Edition Online Edition: 2015/2/17 (Japan time), doi: 10.1002/anie.201411923.
Article link (Publication)
Links
Graduate School of Agricultural and Life Sciences
Department of Biotechnology, Graduate School of Agricultural and Life Sciences
Laboratory of Cell Biotechnology, Biotechnology Research Center (Japanese)