Toward the elucidation of germination-inducing mechanism in witchweed Structure for parasitic weed proteins to bind specific germination compounds

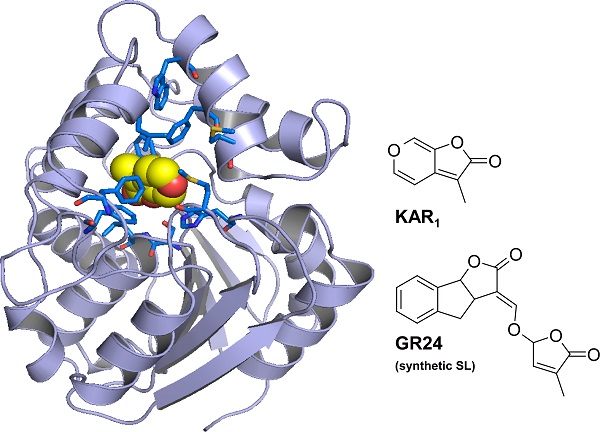
Structure of ShKAI2iB binding karrikin (KAR1)
The blue rods represent the amino acid residues enclosing karrikin in the ShKAI2iB protein. The space enclosed by the blue rods is just the right size for karrikin, but too small to accommodate strigolactone (GR24), which is larger than karrikin.
© 2016 Masaru Tanokura.
Researchers at the University of Tokyo have revealed the underlying structure of a KAI2 protein in parasitic witchweed that predisposes the protein to bind only to karrikin, a germination-inducing compound. The current outcome will likely pave the way for controlling the spread of these root parasitic weeds, which get their nutrients by feeding off the roots of other plants, thereby causing huge losses in agricultural production in Africa and other areas.
Witchweed is a parasitic weed of the genus Striga found widely in sub-Saharan Africa, which infects and causes great damage to important crop cereals. KAI2 proteins are said to detect the strigolactone compounds that induce seed germination, which are secreted by sorghum, corn, rice and other hosts’ roots. Several types of KAI2 proteins are present in Striga, but their response to karrikin and strigolactone varies.
The research groups led by Professor Masaru Tanokura and Professor Tadao Asami, both at the Graduate School of Agricultural and Life Sciences, the University of Tokyo, found that one of those KAI2 proteins, ShKAI2iB, binds only to karrikin. Furthermore, they uncovered through X-ray crystallography the structure that determines karrikin-binding specificity of ShKAI2iB, helping to explain the diversity of KAI2 proteins in Striga at the molecular level. They showed that the narrowing of the space where ShKAI2iB binds to germination-inducing compounds, like karrikin and strigolactones, makes the protein lose its capacity to accommodate strigolactones, which are larger in molecular size than karrikin.
The current research made clear a part of the mechanism that determines specificity in diverse Striga KAI2 proteins to select the particular germination-inducing compounds they ultimately bind with. The study’s findings are likely to help scientists design new chemicals to regulate germination of root parasitic weeds.
“Our research unveiled the structural basis of a protein involved in germination of root parasitic weed Striga binding specific germination compounds” says Tanokura. He continues, “Our findings are expected to provide a clue for further research in designing new compounds of inducing or inhibiting the germination of Striga.”
Press release (Japanese)
Paper
, "Structural basis of unique ligand specificity of KAI2-like protein from parasitic weed Striga hermonthica", Scientific Reports Online Edition: 2016/08/10 (Japan time), doi: 10.1038/srep31386.
Article link (Publication)
Links
Graduate School of Agricultural and Life Sciences
Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences






