Recovery
Radioactive contamination and agriculture
Due to the accident at the Fukushima Daiichi Nuclear Power Station, a vast amount of radioactive material was emitted. Proposing solutions to the problems concerning the treatment of this material is an important subject for academic research. Our job as researchers now is to provide a range of new findings to help people protect themselves from the various environmental risks they face.
Tsuyoshi Miyazaki
Professor, Graduate School of Agricultural and Life Sciences
What will happen to radioactive material that has fallen out of the air and settled on the ground?
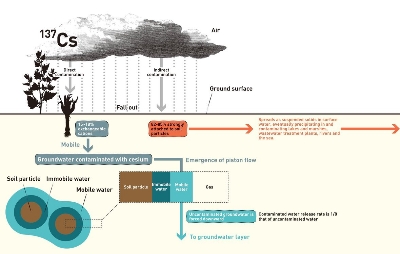
Radioactive material emitted into the air eventually falls to the ground. When this fallout is deposited on the stems, leaves and spikes or ears of crop plants, this is called “direct contamination.” When this material falls onto the soil and is absorbed by crop plants through the roots, the plants are considered to be “indirectly contaminated.” People are now highly concerned about what will happen to the radioactive fallout.
Cesium 137: 80% will be absorbed and the remaining 20% will remain mobile
The movement of cesium 137 fallout began to be clarified by studies on the impact of atmospheric nuclear tests during the 1950s and 1960s. Thanks to the steady efforts of researchers who investigated the impact of the Chernobyl nuclear accident of 1986 it was revealed that in Ukraine, Belarus, and Russia, 75% to 90% of cesium 137 fallout was confined between layers of clay minerals (called “specific adsorption”) and the remaining 10% to 25% remained mobile as exchangeable cations. Moreover Misako Komamura et al. continued to detect cesium 137 fallout in the soil of agricultural land across Japan from 1959 to 2000. The researchers found out that 82% and 85% of the fallout in crop fields and rice paddies, respectively, was confined between the underground layers of clay minerals, while 18% and 15% respectively was floating in soil water and mobile in water as exchangeable cations. Additionally, strontium 90 was found to be more mobile in water than cesium 137 as exchangeable cations.
Specific adsorption of cesium 137 (82%–85%)
Near the ground surface, vast amounts of cesium 137 remains confined in clay mineral particles (Figure 1) but these clay particles will drift away, suspended in ground surface water, and concentrate in rivers, sewage and drains. The particles will then accumulate as sludge in wastewater treatment facilities or eventually drift out the sea and precipitate as sediment near river mouths.
Cesium 137 does not easily detach from clay particles and is therefore detected at high concentrations in sewage sludge and sea bottom sediments. For the period from March 11 to the end of June, the contamination of sludge by radioactive substances was widely reported by the media. This contamination is closely related to the specific adsorption of cesium 137.
Cesium 137 that remains mobile (15%–18%)
As for the cesium 137 exchangeable cations (Figure 1), it is feared that they will contaminate the soil and water to a significant depth. The nuclear tests of the 1950s and 1960s released large amounts of radioactive substances into the air. Researchers conducting on-site measurements of tritium in Denmark found that radioactive substances that had fallen from the air to the land surface in rainwater reached the groundwater layer very slowly (by a factor of around eight) compared with uncontaminated soil water. This was a phenomenon that interestedly me greatly. I therefore conducted some laboratory experiments and performed a theoretical analysis to elucidate its mechanism. As a result, I found that soil water could be classified into two categories: water confined in soil particles (immobile) and water that freely moved through the interstices between particles (mobile). When soil water containing contaminants newly infiltrates into the ground, it pushes the mobile existing uncontaminated soil water downward (called “piston flow”), causing its own mobile contaminants to remain near the surface. The uncontaminated soil water will then reach the groundwater layer. I described this mechanism in my book, Kankyochisuigaku (University of Tokyo Press, 2000). The reason why cesium 137 exchangeable cations tend to stay near the ground surface has therefore already been largely elucidated.
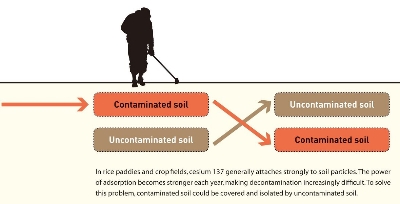
Effectiveness of replacing the surface soil
It is difficult to decontaminate soil, sewage sludge or sea bottom sludge contaminated with cesium 137 by washing it, as cesium 137 does not easily detach from the fine clay particles. Investigations have therefore been conducted on burying the contaminated soil and sewage sludge deep under layers of soil. In order to decrease the gamma rays emitted from cesium 137 contained in soil to one-tenth when measured on the land surface, how many centimeters of uncontaminated soil should be used to cover the contaminated soil? (Figure 2) In order to answer this question, it is necessary to use the mass-absorption coefficients of soil for the gamma rays emitted from cesium. The mass-absorption coefficients of substances provide an important indicator to quantify the attenuation of gamma rays that have passed through the substances. According to my own measurements, the mass-absorption coefficients (cm2/g) for gamma rays emitted from cesium 137 (unidirectional beams) are 0.0768, 0.0764, 0.0756, and 0.0835 for decomposed granite soil, volcanic ash soil, alluvial soil, and distilled water, respectively. We can also predict the degree of attenuation achieved by combining these substances. For example, if the volcanic ash soil contains water at 50% in terms of volume and is 27 cm thick, the rays will be attenuated to one-tenth. In order to achieve the same attenuation effect using water or dried volcanic ash soil alone, the layer needs to be 27.6 cm and 52.9 cm thick, respectively. I strongly recommend the use of mass-absorption coefficients when implementing the decontamination of cesium 137 by soil cover.
Impact on agricultural products
We can assess the indirect contamination of cesium 137 by the use of transfer coefficients, which represent the crop plant’s absorption rates of cesium 137 contained in soil. The Ministry of Agriculture, Forestry and Fisheries publishes the coefficient values, such as 0.0007 for tomatoes and 0.012 for rice. These are placeholder reference values, however, and we at the Graduate School of Agricultural and Life Sciences are working to establish a system to calculate the precise values. As for direct contamination caused by the nuclear accident, a multitude of reports are being made.