Temporal Coding of Insulin Action through Multiplexing of the AKT Pathway

The AKT Pathway Multiplexes Temporal Patterns of Insulin level.© Shinya Kuroda
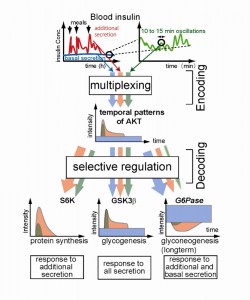
Temporal patterns of insulin secretion (upper panels) are encoded in temporal patterns of AKT. The encoded information on insulin secretion is selectively decoded by downstream target molecules using differences in network structures and kinetics.© Shinya Kuroda
Professor Shinya Kuroda and his research group at the University of Tokyo’s Graduate School of Science have shown that a signaling pathway can multiplex distinct temporal patterns of stimulation, and downstream molecules can selectively decode those patterns.
It is known that blood insulin levels exhibit several temporal patterns, including additional secretion, which is observed in response to meals, basal secretion, which is characterized by persistently low circulating insulin concentrations, and small-amplitude oscillations, which recur approximately every 10 to 15 minutes. The relevance of insulin secretion abnormalities, for example in additional secretion patterns and the 10- to 15-minute oscillations in secretion patterns, in the pathogenesis of type 2 diabetes mellitus has been recognized in some studies as important for optimizing insulin action on target tissues. These studies indicate that insulin selectively regulates metabolic processes depending on temporal patterns of insulin secretion. However, although the importance of temporal patterns of insulin secretion has been reported, the mechanism of its selective and specific regulation of downstream response remained unknown.
Professor Kuroda’s group have shown that the AKT pathway, an important pathway involving the protein kinase AKT and essential for the metabolic functions of insulin, can multiplex distinct temporal patterns of insulin secretion, and downstream molecules such as S6K, GSK3β and G6Pase can selectively decode those patterns. Thus, cells can encode multiple items of cellular information into temporal patterns of molecular concentration and process them using a temporal pattern multiplexing system, so that cells can selectively regulate downstream processes. This research is a huge step forward in our understanding of insulin action and type 2 diabetes. The result suggests the possibility that alterations in the temporal pattern of insulin action may lead to selective impairment of a certain insulin action in a specific target tissue. Signaling for both gluconeogenesis and lipogenesis are exaggerated in the liver of insulin-resistant type 2 diabetes, whereas the former is inhibited and the latter is stimulated by insulin in normal conditions. This apparently paradoxical phenomenon can be explained by the different responses of each insulin target molecule to temporal patterns of insulin stimulation.
Press release (Japanese)
Paper
Hiroyuki Kubota, Rei Noguchi, Yu Toyoshima, Yu-ichi Ozaki, Shinsuke Uda, Kanako Watanabe, Wataru Ogawa, and Shinya Kuroda,
“Temporal Coding of Insulin Action through Multiplexing of the AKT Pathway”,
Molecular Cell46 June 2012: 1-13, doi: 10.1016/j.molcel.2012.04.018.
Article link
Links
Bioinformatics and Systems Biology






