Precise polymerization for the rest of us Toward simple polymer synthesis and recycling

Polymers, one of the principal raw materials of plastic and rubber, are synthesized by connecting small molecules (monomers) in a chain. Optimizing the kinds of monomers used and the number of monomers connected enables the creation of polymers suited to a variety of uses. However, precise control of polymer synthesis requires well-equipped facilities and advanced expertise.
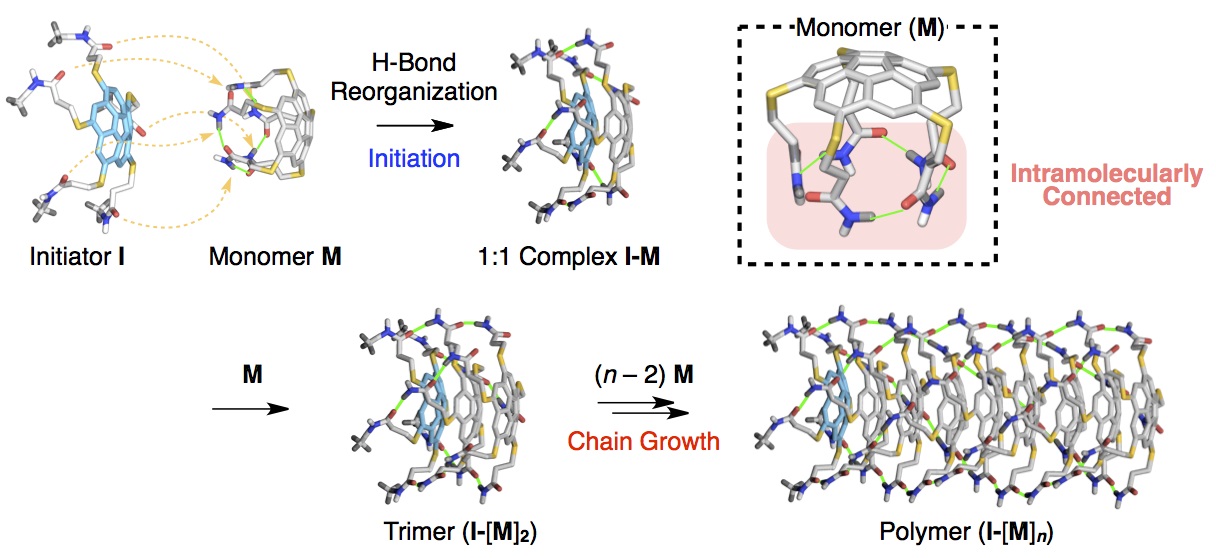
In contrast to conventional polymers, another class of polymers known as “supramolecular polymers” has been developed, the synthesis of which does not entail chemical reactions. Since the monomers for supramolecular polymers are connected through hydrogen bonds and other weak interactions, they can be easily synthesized without any special facilities. However, it has been impossible to precisely control the chain length and order in which monomers are connected to date.
Professor Takuzo Aida’s research group at the University of Tokyo Graduate School of Engineering has developed a novel method to precisely control the synthesis of supramolecular polymers, allowing the creation of polymers of precise length.
Connecting two of the coupling points of the monomers within the molecule to create a ring prevents the monomers from spontaneously connecting to one another. By adding a small molecule with one connecting part (an initiator), a dimer is created containing two the monomers linked in a chain. Monomers continue to join the chain as long as the initiator is present, allowing the length of the chains to be precisely controlled by varying the proportion of monomer and initiator. For example, if the ratio of monomer to initiator is 1,000 to 1, it is possible to create polymers incorporating 1,000 monomers.
This "supramolecular polymer" synthetic method enables the creation of polymers by anyone with a test tube, without requiring the various equipment, advanced technology and experience required for precise polymer synthesis using conventional methods. Furthermore, the weak intramolecular binding force between monomers means that the polymers can easily be decomposed back into their constituent parts, reducing the burden on the environment. Future research will focus on the development of supramolecular polymers with physical properties similar to conventional polymers.
Paper
, "A Rational Strategy for the Realization of Chain-Growth Supramolecular Polymerization", Science Online Edition: 2015/2/6 (Japan time), doi: 10.1126/science.aaa4249.
Article link (Publication)
Links
Graduate School of Engineering
Graduate School of Engineering, Department of Chemistry and Biotechnology
Graduate School of Engineering, Department of Chemistry and Biotechnology, Aida Laboratory