New mechanism of innate immune system activation STING protein is activated at the Golgi apparatus

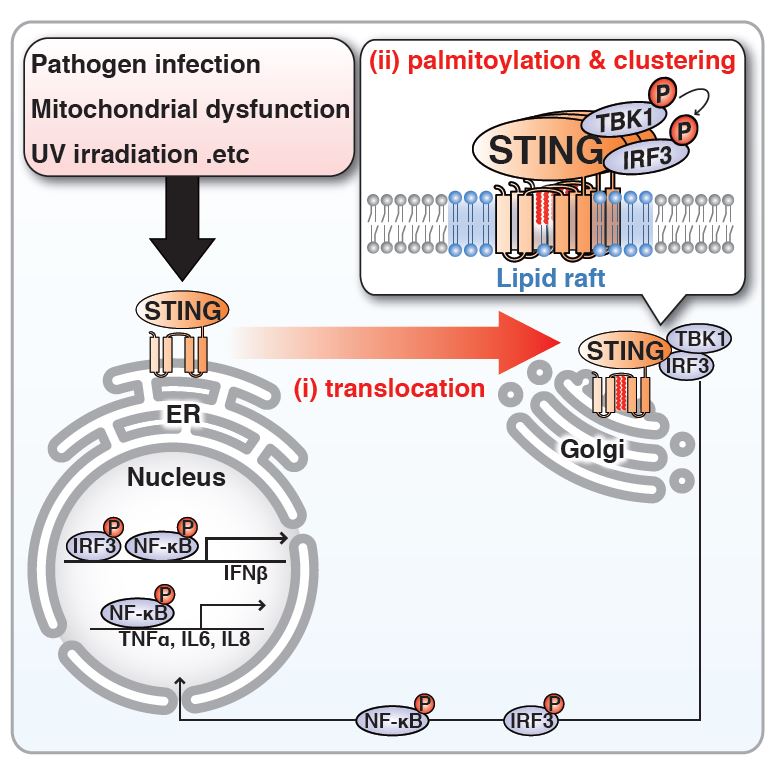
The activation of STING by palmitoylation at the Golgi
(i) STING translocates from the ER to the Golgi upon infection with DNA viruses. (ii) STING is palmitoylated at the Golgi and clusters in the lipid raft at the Golgi, leading to the activation of downstream molecules (TBK1/IRF3).
© 2016 Kojiro Mukai
Researchers at the University of Tokyo have revealed the mechanism underlying the activation of STING, an innate immune sensor that triggers inflammation in order to remove pathogens. This discovery provides novel therapeutic targets for developing treatments for infections and inflammatory diseases.
When cells are infected with foreign matter such as DNA viruses or bacteria, the foreign DNA is sensed by the protein STING embedded in the membrane of the endoplasmic reticulum (ER, an organelle that is an important site of protein production), triggering the release of type I interferon and inflammatory response to eliminate the foreign substance. This essential basic cellular response is part of the innate immune system that recognizes and eliminates pathogens from our bodies. However, it was unclear why the transmembrane protein STING responded to foreign DNA. Additionally, although it is known that STING translocates from the ER to a location close to the nucleus when it detects foreign DNA, the role of this translocation remained unknown.
In this study, the research group of Assistant Professor Kojiro Mukai, Associate Professor Tomohiko Taguchi and Professor Hiroyuki Arai at the University of Tokyo Graduate School of Pharmaceutical Sciences found by cell biological analysis that (i) STING is activated at the Golgi (an organelle involved in protein transport within the cell), not at the ER, and (ii) the activation of STING requires its palmitoylation (a type of protein modification) at the Golgi. The unique lipid environment of the Golgi is also found essential for the activation of STING.
In various pathogenic conditions such as autoimmune diseases, cancer, mitochondrial dysfunction, and UV irradiation, STING is often activated, causing an aberrant inflammatory response. Thus, the findings offer new opportunities to treat such inflammatory diseases by suppressing the palmitoylation of STING or the manipulation of the Golgi lipid composition.
“This study revealed the importance of membrane phospholipids of organelles in the innate immune response and provides clues to the development of therapies for infectious diseases, immune diseases, and cancers,” says Arai.
Paper
, "Activation of STING requires palmitoylation at the Golgi", Nature Communications: 2016/06/21 (Japan time), doi: 10.1038/ncomms11932.
Article link (Publication)
Links
Graduate School of Pharmaceutical Sciences
Department of Health Chemistry, Graduate School of Pharmaceutical Sciences