Creation of novel multifunctional oxidation catalyst Step toward rational redesign of biosynthetic drug development
 Creation of novel multifunctional oxidation catalyst
Creation of novel multifunctional oxidation catalystCrystal structure-based mutagenesis analysis enables creation of novel oxidation catalysts
© 2018 Ikuro Abe.
Researchers at the University of Tokyo have succeeded in the functional conversion of multifunctional dioxygenases through an enzyme engineering study based on crystal structure analysis. Through this study, they created a novel oxidation enzyme, which catalyzes additional oxidation compared with wild types of enzyme. These engineered dioxygenases expand the bioactive meroterpenoid library and contribute to medicinal chemistry.
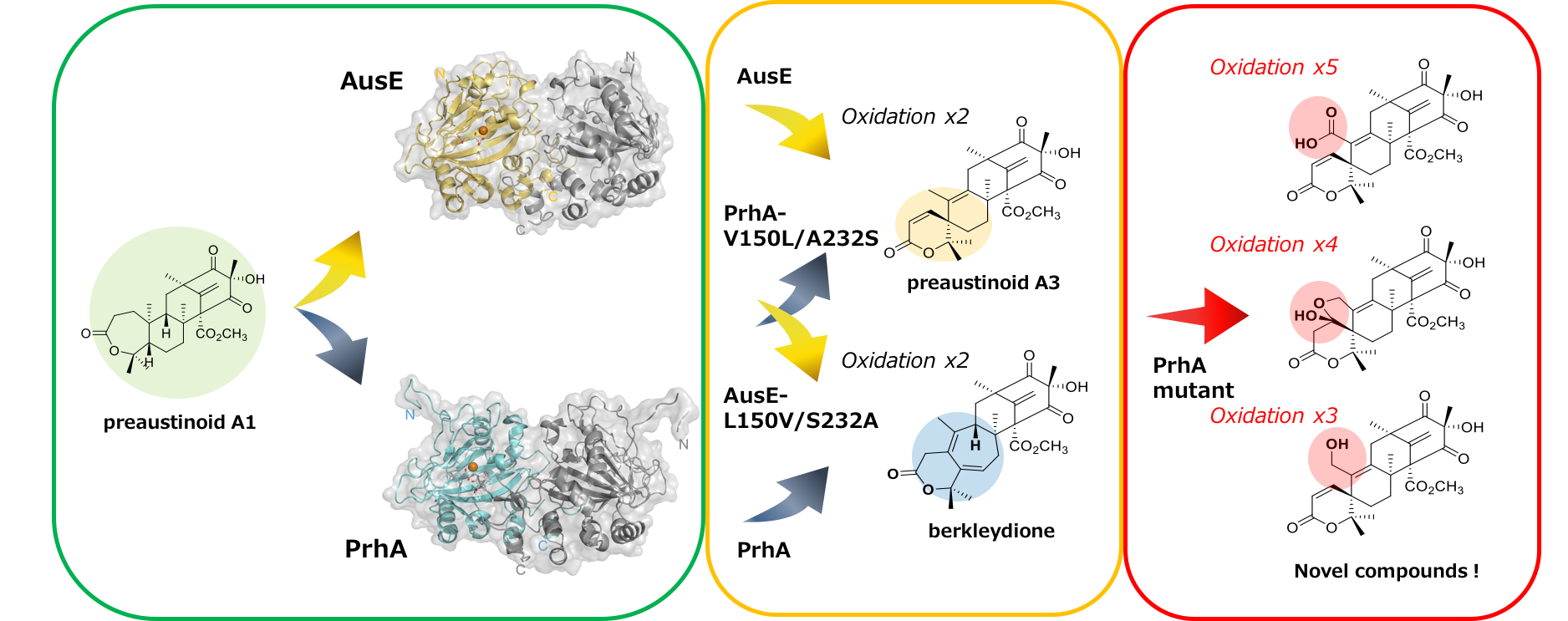
Non-heme iron, alpha-ketoglutarate (αKG)-dependent dioxygenases catalyze key oxidation reactions to afford complex molecular structures in fungal meroterpenoid biosynthesis. AusE and PrhA are two dioxygenases that catalyze intriguing oxidative rearrangement reactions in austinol and paraherquonin biosynthetic pathways, respectively. They have high sequence similarity (as high as 78 percent) and both enzymes accept the same preaustinoid A1 substrate, but generate different products, preaustinoid A3 in AusE and berkeleydione in PrhA.
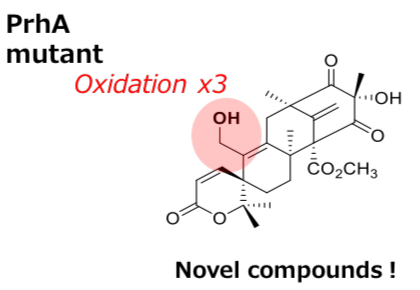
The research group of graduate student Yu Nakashima and Professor Ikuro Abe at the University of Tokyo’s Graduate School of Pharmaceutical Sciences has described the X-ray crystal structures of apo AusE and PrhA, as well as PrhA complexed to iron (II), αKG and preaustinoid A1 at under 2.1 angstrom resolution. Comparison of the crystal structures of AusE and PrhA identified differences in several key active site residues proximal to the substrate. Mutation of the identified PrhA active site residues to the respective residues in AusE (A232S/V150L) resulted in a PrhA-A232S/V150L mutant that catalyzes an AusE-type reaction to produce preaustinoid A3. The researchers also generated a PrhA-A232S/V150L/M241V triple mutant and found that this generates several novel compounds through four-step oxidation from preaustinoid A1. This study presents the structural basis of a novel reaction mechanism of multifunctional dioxygenases, in combination with mutagenesis studies that successfully led to the expanded function for these enzymes.
Abe explains, “In this study, we developed a novel oxidation catalyst, which produced a complex structure of meroterpenoids.” He continues, “Furthermore, our results could pave the way for construction of a bioactive meroterpenoid library contributing to medicinal chemistry.”
This work was supported by the Grant-in-Aid for Scientific Research on Innovative Areas “Creation of Complex Functional Molecules by Rational Redesign of Biosynthetic Machineries” and the Strategic International Collaborative Research Program between the Japan Science and Technology Agency (JST) and the National Natural Science Foundation of China (NSFC) titled “Exploitation of the cryptic secondary metabolites from plant microbiome through biological interaction.”
Papers
Yu Nakashima, Takahiro Mori, Hitomi Nakamura, Takayoshi Awakawa, Shotaro Hoshino, Miki Senda, Toshiya Senda, and Ikuro Abe, "Structure function and engineering of multifunctional non-heme iron dependent oxygenases in fungal meroterpenoid biosynthesis," Nature Communications: January 9, 2018, doi:10.1038/s41467-017-02371-w.
Link (Publication )
)
Related links
- Graduate School of Pharmaceutical Sciences

- Laboratory of Natural Product Chemistry, Graduate School of Pharmaceutical Sciences

- Grant-in-Aid for Scientific Research on Innovative Areas “Creation of Complex Functional Molecules by Rational Redesign of Biosynthetic Machineries”






