UTokyo team's own X-ray method finds new applications First dynamic observations of aggregated proteins excited at the single-molecule level
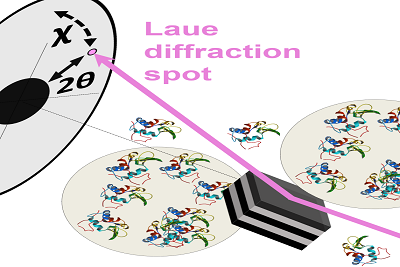
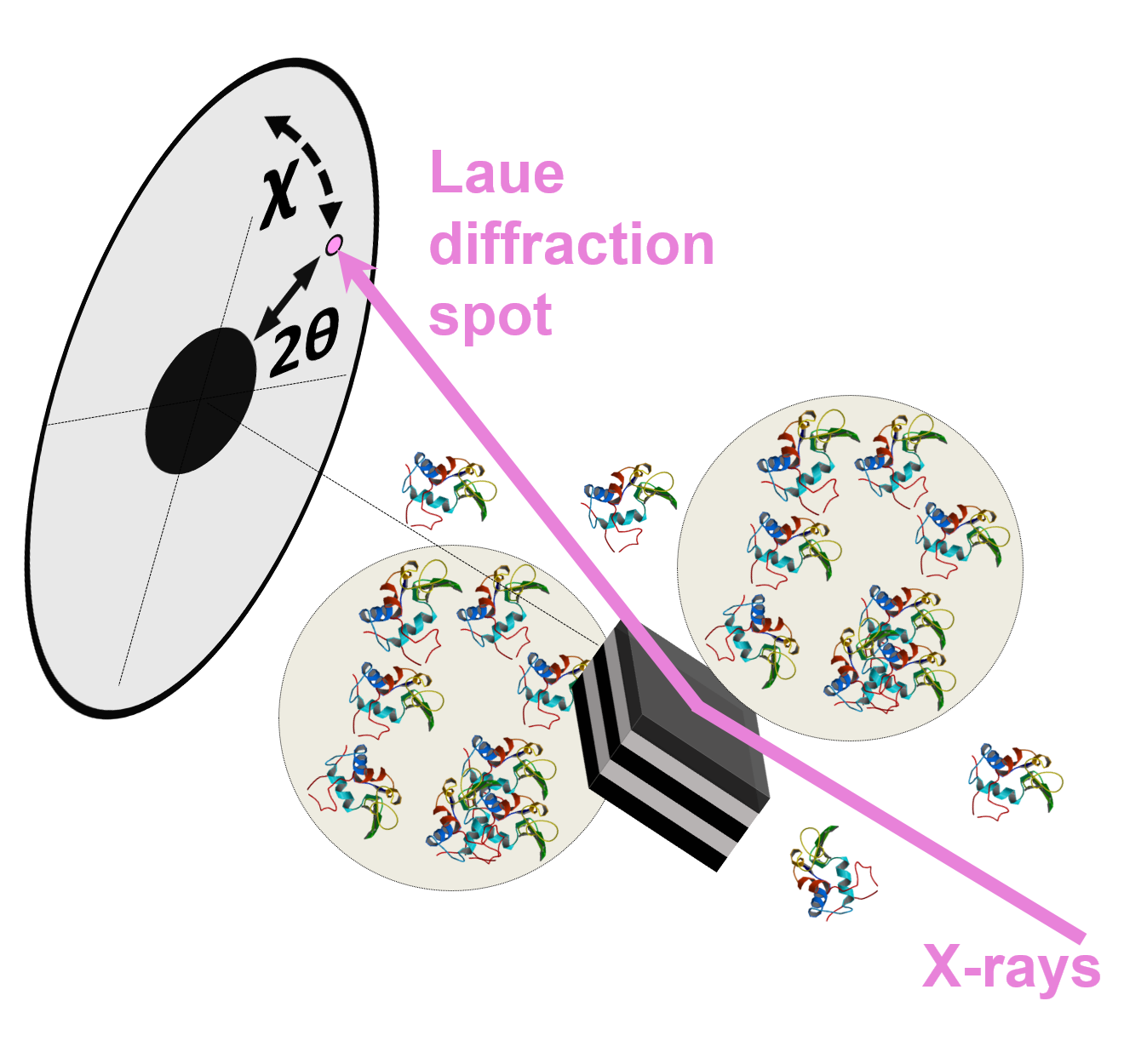
A measurement model in supersaturated solution state of X-ray single-molecule tracking method (DXT) realizing network measurement of aggregated protein molecules
© 2018 The University of Tokyo.
Previously, researchers had no method to observe the movements of a single molecule within a supersaturated solution. A research group led by Professor Yuji Sasaki from the University of Tokyo Graduate School of Frontier Sciences has used a superhigh-sensitivity single-molecule measurement method called diffracted X-ray tracking (DXT) in a novel way to collect data that reveal how proteins move in saturated solutions.
As part of the DXT method, chemically labeled gold nanocrystals with a diameter of 20 nanometers are placed at target positions on protein molecules. High-speed time-division tracking is possible from X-ray diffraction observations. Sasaki's research group demonstrated the DXT method for the first time in 1998.
Currently, DXT is recognized as the world's highest-precision and highest-speed single-molecular-dynamics measurement technology for the measurement of an individual molecule on its own. This research result, published in the November 2017 edition of Scientific Reports, is the first time DXT was applied to the measurement of nanoscopic protein dynamics under the supersaturated conditions. Specifically, Sasaki's research group conducted measurements under the protein-solution condition researchers suspect has crystallization precursors not confirmed by experiments, and successfully measured the rotational dynamics of single particles of gold nanocrystals labeled to protein molecules with high accuracy and at high speed. DXT experiments were conducted on a beamline (BL 40 XU) that can utilize high-brightness quasi-monochromatic X-rays of the large synchrotron radiation facility SPring-8.
From this experiment, it was confirmed that the nanocrystal kinetic rotation speed had a peak at 3.7 milliradians (mrad) in the stable protein-solution state on the microsecond time scale, but a new 9.5-mrad peak appeared when proteins became aggregated. This result suggests the existence of an ion network structure under solute-dense conditions, which was confirmed by Sasaki's group in the excitation movement with the inorganic substance discovered in 2015. This ion network structure is not only inorganic and organic; researchers confirmed the dynamic characteristics common to protein molecules. Also, when converting this interesting Brownian movement as the force applied to the gold nanocrystal, researchers also confirmed that a weak force field called femtonewton is involved. Researchers suspect that this dynamic aggregation phenomenon is an important physical phenomenon that can maintain the solution state without crystallization by vigorous movement of protein molecules under a high-concentration condition that can be precipitated.
Papers
Y. Matsushita, H. Sekiguchi, JW. Chang, M. Nishijima, K. Ikezaki, D.Hamada, Y. Goto, Y.C. Sasaki, "Nanoscale Dynamics of Protein Assembly Networks in Supersaturated Solutions," Scientific Reports: November 1, 2017, doi:10.1038/s41598-017-14022-7.
Link (Publication )
)
Related links
- Graduate School of Frontier Sciences

- Department of Advanced Materials Science, Graduate School of Frontier Sciences

- Y. C. Sasaki Laboratory, Department of Advanced Materials Science, Graduate School of Frontier Sciences






