How nutrition controls tissue maintenance Amino acid influences health of gut cells
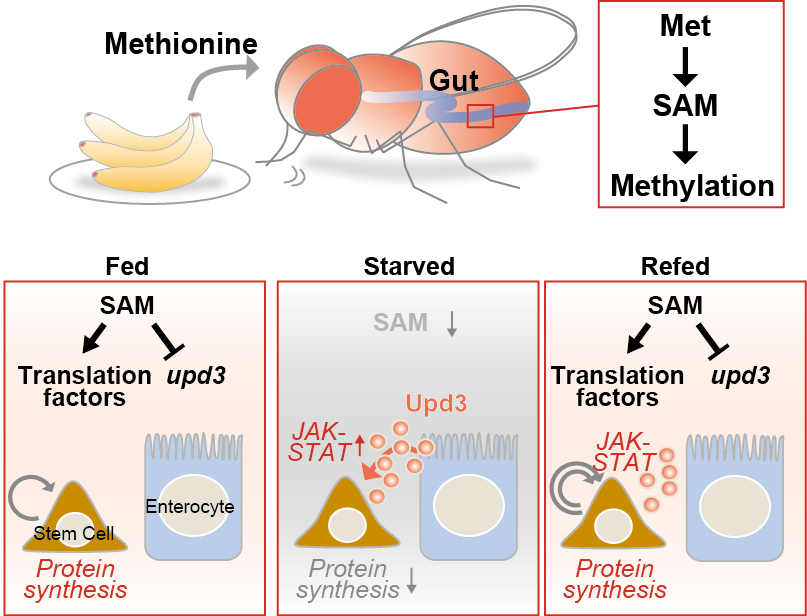
In the normal fed condition, dietary methionine is catalyzed to S-adenosylmethionine (SAM) that regulates translation in the intestinal stem cell (ISC). In the starved condition, SAM depletion reduces translation and proliferation of ISC. Simultaneously, SAM depletion leads Upd3 secretion in enterocyte cells. The activation of the JAK-STAT signaling pathway in ISC by Upd3 and up-regulation of translation can drive rapid division of ISC after refeeding.
© 2018 Kayoko Tsuda-Sakurai.
A team of researchers at the University of Tokyo has demonstrated that S-adenosylmethionine, the primary metabolite of dietary methionine, functions as a key molecule of intestinal homeostasis in fruit flies.
Dietary nutrients and their metabolites are indispensable for tissue homeostasis and individual health. Homeostasis is maintained by constitutive renewal of cells driven by proliferation and differentiation of tissue stem cells. However, the effect of diet on controlling tissue stem cells is complicated and much remains unknown.
The research team of Lecturer Fumiaki Obata, postdoctoral fellow Kayoko Tsuda-Sakurai and Professor Masayuki Miura at the Graduate School of Pharmaceutical Sciences identified S-adenosylmethionine (SAM) as a critical molecule that regulates intestinal stem cell division in Drosophila, or common fruit fly often used for scientific inquiry. SAM can affect the function of target molecules by contributing to their chemical modification.
In their scientific study, the researchers demonstrated that SAM governs intestinal stem cells by controlling the synthesis of proteins by regulating their translation through methylation of protein translation factors. In contrast, SAM in nutrient-absorptive enterocytes, a type of cell in the intestinal epithelium, regulates the production of a cytokine protein called Unpaired 3, which is required for rapid division of intestinal stem cells after refeeding.
The study revealed cell type-specific function of SAM as a key regulator of nutritional control for intestinal homeostasis. Therefore, the research team recommends that studies of amino acid metabolism in each unique cell type may lead to more effective strategies for clinical intervention.
"It was a complicated, time-intensive study, but we finally found a new nutrition-based control mechanism of stem cells and I am glad that we could collect such convincing data," said Miura. He continues, "From this study, we could start to see a smart control mechanism of tissue cell quorum as stem cell proliferation and differentiation by SAM in the intestines. I hope that our results will lead to the research of precise control mechanisms of tissue renewal by amino acid metabolism."
Papers
Fumiaki Obata, Kayoko Tsuda-Sakurai, Takahiro Yamazaki, Ryo Nishio, Kei Nishimura, Masaki Kimura, Masabumi Funakoshi, and Masayuki Miura, "Nutritional control of stem cell division through S-adenosylmethionine in Drosophila intestine.," Developmental Cell Volume 44, Issue 6, p741–751.e3: March 26, 2018, doi:10.1016/j.devcel.2018.02.017.
Link (Publication )
)
Related links
- Graduate School of Pharmaceutical Sciences

- Department of Genetics, Graduate School of Pharmaceutical Sciences






