Learning the loops Study of looped, rotating proteins opens possibilities for drug discovery
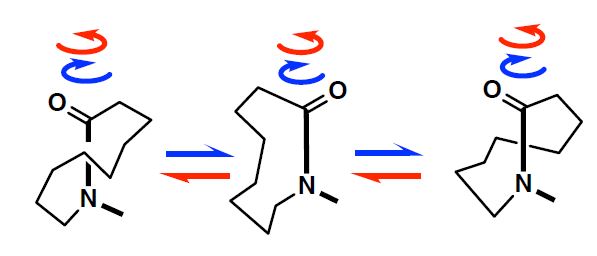
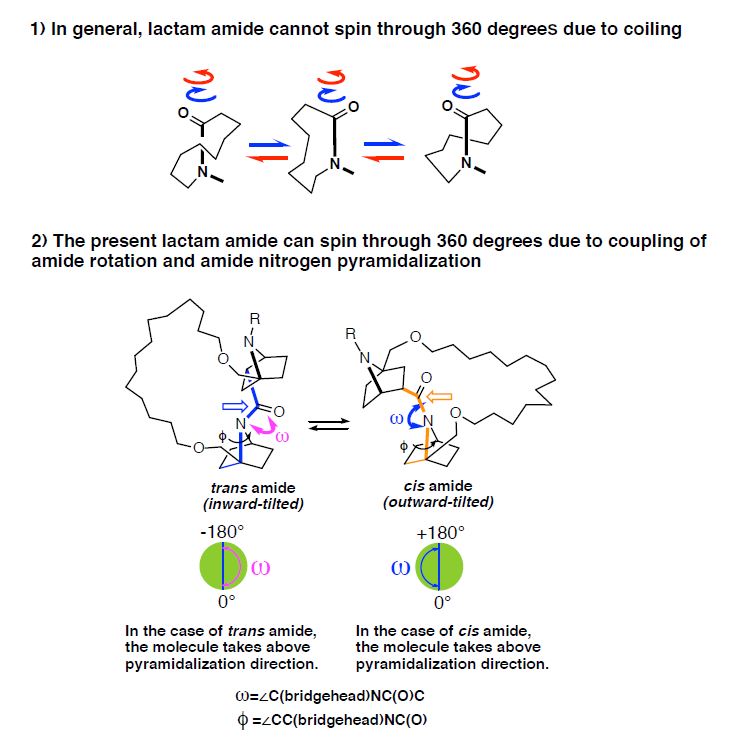
1) In general, lactam amide cannot spin through 360 degrees due to coiling. 2) The present lactam amide can spin through 360 degrees due to coupling of amide rotation and amide nitrogen pyramidalization.
© 2019 Tomohiko Ohwada.
Researchers have uncovered the structural movements of a common class of molecules. The results will provide new methods to analyze bioactive molecular structures, including the design of new medications.
Molecules that contain a particular structural unit are known as amides and are common in nature. Human hair and synthetic materials such as nylon and Kevlar are all built using different types of amides. Many drugs are amides, including the painkiller paracetamol (sold under the brand Tylenol in the U.S. and other parts of the world) and the antibiotic penicillin. Amides have some flexibility in their structure, but no research group was previously able to study their movement. Understanding how amides rotate could allow researchers to unlock even more potential uses of these molecules.
The research team from the University of Tokyo Graduate School of Pharmaceutical Sciences studied a subgroup of amides with structures that include a loop, so-called cyclic lactam amides. The goal of the research was to design and build a lactam amide that could rotate a full 360 degrees.
“If we want to plan drug discovery projects using cyclic proteins, we must determine the solution structure and bioactive structures – or those having an effect on living tissue – of cyclic peptides (protein fragments),” said Lecturer Yuko Otani, the first author of the research publication.
Researchers observed the lactam rotation using nuclear magnetic resonance spectroscopy, a specialized technique to measure the local magnetic fields around the center of atoms. Researchers also used computational simulations to monitor the rotation of lactam amides.
The results revealed that lactam amides can spin through 360 degrees, as in open-chain amides. This is an unexpected result because lactam amides cannot spin through 360 degrees due to ring coiling and high rotational barriers. The amides have cis and trans isomerization characterized by the same molecular formula but a different arrangement of the atoms spatially. The researchers utilized a nonplanar amide. As the chain length increased, the rotational rate of trans to cis lactam amide decreased, and consequently the trans ratio increased.
“When we spoke about this research project at a recent scientific meeting, we received many comments from other researchers along the lines of, ‘Lactam amide rotation is totally new for me.’ We’ve been involved in this amide rotation project for over five years, so it’s always unexpected to be reminded that this research is still so uncommon in the wider scientific community,” said Professor Tomohiko Ohwada, the leader of the Laboratory of Organic and Medicinal Chemistry where this research was performed.
This work was supported by the Japan Society for the Promotion of Science.
Papers
Yuko Otani, Xin Liu, Hisashi Ohno, Siyuan Wang, Luhan Zhai, Aoze Su, Masatoshi Kawahata , Kentaro Yamaguchi, Tomohiko Ohwada, "Amide nitrogen pyramidalization changes lactam amide spinning," Nature Communications: January 28, 2019, doi:10.1038/s41467-018-08249-9.
Link (Publication )
)
Related links
- Graduate School of Pharmaceutical Sciences

- Laboratory of Organic and Medicinal Chemistry, Graduate School of Pharmaceutical Sciences






