Better mouse model developed to study immune response Genetically modified mouse provides higher quality data in first study
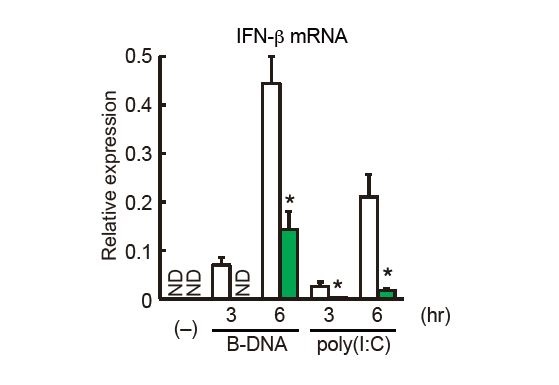
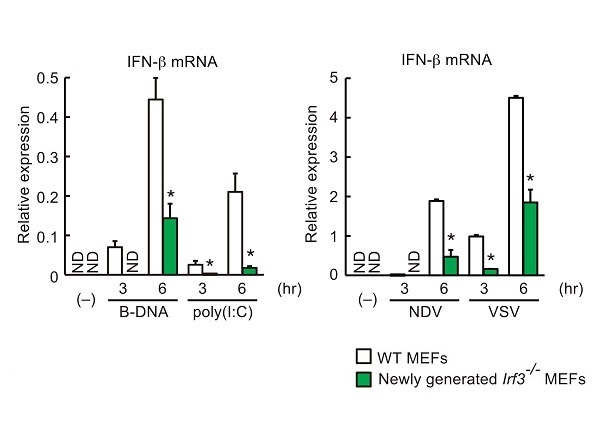
Researchers engineered Irf3 conditional knockout mice to allow for the cell-type or tissue-specific ablation of Irf3 (left panel). Type I interferon gene induction by Newcastle disease virus (NDV) or vesicular stomatitis virus (VSV) infection was profoundly reduced in Irf3-deficient dendritic cells (right panel, green bar).
© 2018 Hideyuki Yanai.
University of Tokyo researchers and their collaborators have demonstrated that a transcription factor called interferon regulatory factor 3 (IRF3) is a specific and essential regulator of innate immune responses that are evoked by pathogenic viruses and bacteria. Scientific understanding of the regulation of host defense to pathogens and IRF3 has taken a further step forward with the availability of Irf3flox/flox, a gene that allows for tissue-specific Irf3 genomic deletion.
The innate immune system specializes in detecting and orchestrating appropriate responses to pathogens. A key mechanism that the innate immune system uses to transmit and amplify warning is the secretion of cytokines, such as the type I interferons IFN-α and IFN-β. The direct transcriptional regulation of a number of critical and potent immune mediators is how IRF3 plays a crucial role in the innate system. Many previous studies characterizing IRF3’s role in regulating innate immune signaling and immune cell death used conventional Irf3-deficient mice. However, in addition to lacking tissue specificity, the scientific community discovered that these conventional mice also had an extra gene deleted.
A research group led by Project Associate Professor Hideyuki Yanai at the Institute of Industrial Science engineered Irf3flox/flox mice to study IRF3 function in a tissue- and cell-selective manner. These mice were also used to validate the dependence of IRF3 on innate immune responses. Specifically, Yanai and his colleagues showed that IRF3 was required for the induction of type I interferon messenger RNA and for survival after viral infection. The group identified that IRF3 specifically in myeloid cells, a type of blood cell found in bone marrow, is involved in mediating toxic shock as demonstrated by reduced mortality of CD11bcre/+IRF3flox/flox mice challenged with lipopolysaccharide (LPS)-induced shock, a symptom of bacterial infection. These results indicate that IRF3 is a critical transcription factor for antiviral innate immune responses and inflammatory diseases, and the new Irf3flox/flox lab mouse will serve as a useful tool to explore the activity of IRF3 within multicellular immune responses.
“There have been numerous reports indicating the role of IRF3 in host defense against infection by viruses and bacteria,” said Yanai. He continued, “It is quite interesting and important to clearly demonstrate that this transcription factor is actually important in innate immune responses against pathogen infection by using the conditional Irf3-deficient mice newly generated in this project.”
The current study was carried out in collaboration with Researcher Kohei Kometani and Professor Rudolf Grosschedl from the Max Planck-The University of Tokyo Center for Integrative Inflammology.
Papers
Yanai H, Chiba S, Hangai S, Kometani K, Inoue A, Kimura Y, Abe T, Kiyonari H, Nishio J, Atarashi N, Mizushima Y, Negishi H, Grosschedl R, Taniguchi T., "Revisiting the role of IRF3 in inflammation and immunity by conditional and specifically targeted gene ablation in mice," Proceedings of the National Academy of Sciences of the United States of America : April 30, 2018, doi:10.1073/pnas.1803936115.
Link (Publication )
)





