Cracking the mystery of how we are formed from a single fertilized egg

© frenta / Adobe stock
How is the human body created from a single fertilized egg?
The cell divides into two, then four and eight … eventually forming organs and ultimately becoming a human body. But as the process of embryonic development unfolds inside the womb, much of its mechanism still remains a mystery. What’s occurring inside the uterus when the embryo is implanted in the uterine wall? How do cells differentiate and develop into individual organs?
Seeking to find answers to such questions, Dr. Ayaka Yanagida, a research associate at the Graduate School of Agricultural and Life Sciences, is working to replicate some of the processes of embryonic development in a test tube. Using stem cells ― unspecialized cells in our body that can differentiate into any type of body cell ― Yanagida aims to observe and study what’s happening during the early stages of embryonic development.
“I’m very interested in understanding how I was created from a single fertilized egg. I want to understand what’s happening inside the mother’s body and how an embryo develops,” said Yanagida, who was selected as a University of Tokyo Excellent Young Researcher in 2022. She maintains that unlocking the “black box” could help uncover the causes of miscarriage or infertility, which, according to a World Health Organization 2023 report, an estimated 1 in 6 adults experience worldwide. And suffice to say, pinning down the causes could also lead to the development of treatments, Yanagida pointed out.
Conceiving a baby mouse without egg or sperm
Back in 2004, when Yanagida entered the University of Tokyo as an undergraduate student, she was interested in studying parasites. Finding the organisms’ various shapes and features interesting, Yanagida knocked on the door of a laboratory in the Department of Veterinary Medical Sciences where she could study parasites. But she was not selected through a lottery to decide the incoming lab members, so she couldn't get in. Instead, she joined the lab of then-Associate Professor Kazuhiko Imakawa, who specializes in animal reproduction. There, while learning about bovine reproduction and how the placenta is formed, she worked alongside a researcher who was at the lab during his yearlong sabbatical. That experience assisting the visiting researcher, Yanagida said, later led her to work on research related to studying the wonder of how a fully formed human body develops.

“We were trying to create a mouse fetus without using sperm and egg. We created a fertilized egglike structure from stem cells and implanted that in the body of a female mouse to see if it could develop into a fetus,” Yanagida said. “The research was very interesting. It was an area that was largely unknown back then, but I think many researchers around the world were plugging away quietly with the aspiration of creating a fetus.”
To Yanagida’s surprise, one day, a female mouse involved in the experiment gave birth to mice babies. “I was so excited thinking that I was witnessing a phenomenon that was being observed for the first time in the world. It was a kind of excitement that I never felt before,” Yanagida said, recalling the moment she saw the newborn mice.
What she later learned, however, was that there was an error in the experiment and the babies were born as a result of normal mating. “The female mouse was supposed to mate with a vasectomized male mouse to induce pseudopregnancy — a state in which the female body thinks it’s pregnant, when it isn’t, and undergoes changes consistent with pregnancy. We were to then implant fertilized egglike structures made from stem cells into the female mouse,” Yanagida explained. “But we found out that the vasectomy was unsuccessful and the babies were born as a result of normal mating.” Regardless of this failure, Yanagida said the sensation she felt when she saw the baby mice has stayed with her ever since and has remained a driving force behind her research.
After developing a strong interest in the “beginning of life” of a human organism, Yanagida entered the Graduate School of Medicine to conduct embryo-related research. She joined the laboratory of then-Professor Hiromitsu Nakauchi, who has been leading a project to grow human organs in animals, and engaged in research on liver regeneration using induced pluripotent stem (iPS) cells and embryo manipulation, among other studies. After receiving a doctorate and working as a postdoctoral fellow for a year, she moved to the University of Cambridge in the U.K. There, Yanagida was involved in embryo and stem cell research, and subsequently from the fourth year, she also started working on creating a blastocyst — a ball of cells that develops into the fetus and placenta — using human stem cells. A study on embryo shapes, the culmination of her six years’ research in the U.K., was published in the scientific journal Cell in 2022.
“There are two types of cell layers inside a blastocyst — an epiblast, which develops into the body, and the primitive endoderm, which provides nutrition to the embryo. Their cells are known to coexist initially in the same mixture. But they separate into two layers before the blastocyst attaches to the uterus. I studied how the two types of cells divide,” Yanagida explained when talking about the study she worked on for six years. “It was a phenomenon whose mechanism had not been elucidated for a long time.”
Creating an embryo model from stem cells
Yanagida is currently engaged in a study to visualize the process of embryo implantation and its early development. As the use of a human embryo for research is fraught with ethical issues, Yanagida is trying to create a “model” from human stem cells that behaves much like a human embryo and mimics the biological processes of embryonic development in a test tube.
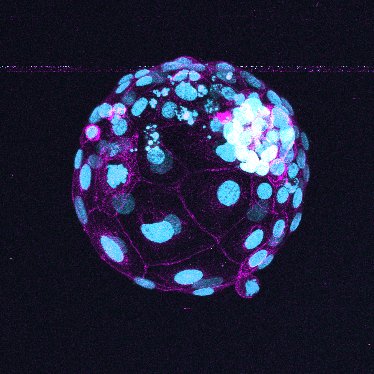
When a blastocyst attaches to the uterus, the blastocyst’s outer cell layer that develops into the placenta grows and starts releasing hormones. But even after the trophectoderm, as the cell layer is called, develops and pregnancy can be confirmed, about 15% of pregnancies are said to result in miscarriage. Does the cause of the failed pregnancy lie in the way the blastocyst is attached to the uterus? Or is the direction of the attachment a critical factor? Yanagida hopes to find out what’s happening in the uterus, using the embryonic model she’s creating to observe the early development of an embryo.
“We have a good understanding of the development of a fertilized egg up until the stage of implantation because we can observe it in a test tube. But we don’t know what happens inside the mother’s body in the stages between implantation and the subsequent development of organs,” Yanagida said.
By replicating the process in the lab, she hopes to understand the mechanism of normal embryonic development and to clarify the causes of abnormalities.
The chapter of truly enhancing our understanding of implantation has just begun, Yanagida said. “Research on mimicking human embryonic development in a test tube has made great progress in the past year or two. There have been reports of embryonic development progressing after ‘pseudo-implantation’ in a lab. But compared to actual human cells, the lab-grown cells overall are different, even if there are partial similarities,” she explained.
Yanagida said the joy of research is to be able to experience her own growth every day, such as to learn to do things she couldn't do before or make small discoveries. Explaining that she is the type of person who wants to devote herself as long as time allows to her research, she said spending long hours in the lab has never been an issue. Such passion stems from her curiosity as a researcher and her desire to “make the world a better place.”
“I want to come to understand the mystery of life and how we are created. I also want to understand the causes of abnormal embryonic development,” she said. “In the long run, I hope to create a platform where researchers across different academic disciplines can gather and collaborate on research that will lead to treatments for infertility, and other discoveries and solutions.”






