Why do air fresheners only eliminate unpleasant odors?

An introduction to academia starting with “Why?”
After making a list of questions which would pique anyone’s interest, we asked faculty members on campus who might be knowledgeable in these areas to answer them from the perspective of their respective expertise. Let’s take a look into the world of research through questions that you feel you know something about, but cannot answer definitively when actually asked.
Q12. Why do air fresheners only eliminate unpleasant odors?
Scented air fresheners are widely used, especially in restrooms, in Japan. If you think about it, it’s strange that these products can selectively eliminate unpleasant odors and leave the pleasant fragrance intact. How does that work? Answered by Yuki Nakamura
Answered by Yuki NakamuraAssistant Professor, Graduate School of Arts and Sciences
| Synthetic Organic Chemistry |
Eliminating unpleasant odors with masking, chemical reactions and adsorption

The receptor cells in our nasal cavity act as sensors, transmitting the stimulus they receive to the brain when odor-emitting molecules attach to them. If many of the attached molecules are those with pleasant odors, molecules with unpleasant odors have less chance to bind to the sensors, which makes it difficult for us to perceive unpleasant odors. This effect, known as masking, is what’s used by scented air fresheners. They use pleasant-smelling molecules to mask our sensors. The other two main methods are (1) alteration of the chemical structure of molecules emitting unpleasant odors, and (2) physical trapping of molecules inside the pores of adsorbent, which is also known as the porous material. An example of the first chemical reaction method is the use of an acidic substance to neutralize a basic compound, such as ammonia, a molecule often found in our urine that causes an unpleasant smell. As another example, other typical unpleasant odors in restrooms, resulting from hydrogen sulfide and other sulfuric compounds, can be eliminated by adding metallic compounds to form metal sulfides. The second adsorption method, on the other hand, makes use of porous materials such as activated charcoal (also known as activated carbon) and zeolite, which have numerous tiny pores on their surface. These pores can adsorb the odor-causing molecules and prevent them from escaping back into the air.
Most commercial air fresheners use a combination of these methods of masking, chemical reactions and adsorption. Stationary types such as deodorizing beads are likely to have a larger proportion of adsorption porous materials, while sprays will have more compounds that facilitate chemical reactions to alter the molecular structures; however, the exact proportions are not shown on product labels. The ingredients and their blends are developed by each manufacturer, and are most likely to be trade secrets.
In addition to ingredients for eliminating unpleasant odors, air fresheners contain their own pleasant fragrances. How come these scents do not get eliminated along with the unpleasant odors? The reason is that while sulfur in hydrogen sulfide and sulfuric compounds, nitrogen in ammonia, and other elements that cause unpleasant odors can readily attach to the pores in porous materials, fragrance-emitting hydrocarbons such as limonene and other terpenes do not get attached so easily due to the difference in their chemical properties. Of course, there are many molecules that emit pleasant odors that can also be easily adsorbed to the pores, but air freshener manufacturers simply avoid selecting such molecules for their products.
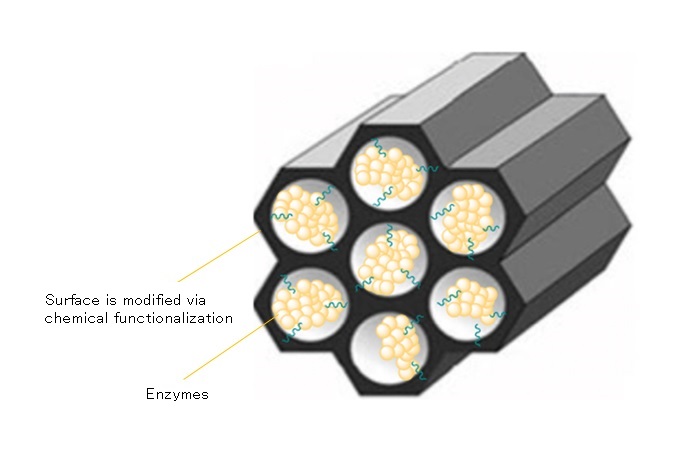
As well as their adsorptive properties, porous materials are known for their ability to stabilize unstable compounds by immobilizing them inside their pores. My research interest is to apply this property to introduce enzymes that often possess sensitive physical properties within the robust structure of porous materials – encasing them in armor, as it were – so the enzymes can be utilized as solid catalysts. I am especially interested in investigating the novel functions and reactivities of these “armored enzymes.” Breaking strong chemical bonds and creating new bonds are usually achieved through application of heat and/or highly reactive reagents to push the reactions forward, but enzymes can smoothly facilitate reactions at ambient temperatures under mild conditions. Another key advantage is that they can be extracted from sources such as plants and fungi, which makes them more readily available than the precious rare metals that are often utilized as powerful catalysts. As for the porous material, I currently focus on the use of mesoporous silica, which has pores of a size that can easily incorporate enzymes. In the future, I hope we’ll be able to recycle the raw materials needed for organic synthesis from sources like waste plastics and biomass.
* This article was originally printed in Tansei 45 (Japanese language only). All information in this article is as of September 2022.






