Ammonia is a chemical essential to many agricultural and industrial processes, but its mode of production comes with an incredibly high energy cost. Various attempts have, and are, being made to produce ammonia more efficiently. For the first time, a group including researchers from the University of Tokyo combined atmospheric nitrogen, water and sunlight, and using two catalysts, produced sizable quantities of ammonia without a high energy cost. Their processes mirror natural processes found in plants utilizing symbiotic bacteria.
You’ve probably heard of ammonia, especially in relation to agriculture, where it’s an essential component of the fertilizers that feed the crops upon which all our lives depend. But here are some numbers to paint a picture of why ammonia is so important and impactful: Just under 200 million tons of ammonia are produced yearly, and 80% of this is used for fertilizer. Also, its production accounts for around 2% of the world's entire energy consumption and correspondingly around 2% of the world's entire carbon dioxide emissions. With these things in mind, it’s understandable why researchers around the world are trying to create a cleaner, more efficient means to produce ammonia.
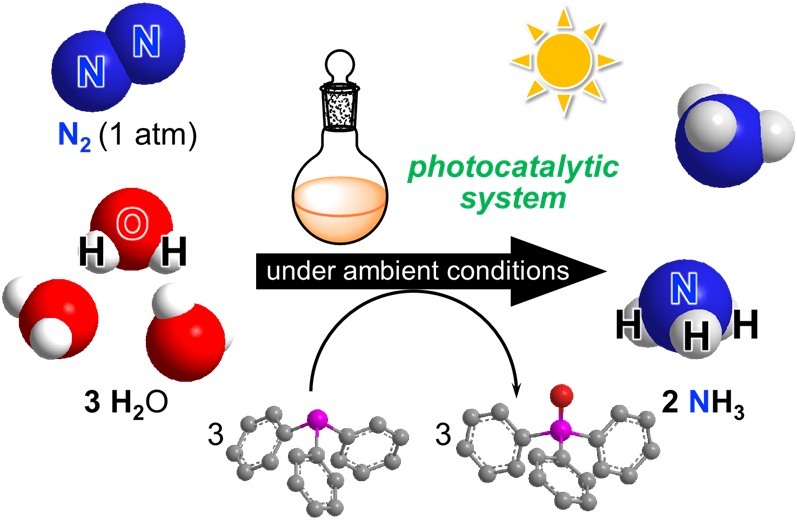
Professor Yoshiaki Nishibayashi from the Department of Applied Chemistry at the University of Tokyo and his team have recently made significant strides in this goal. They succeeded in developing a novel catalytic system for producing ammonia from abundant molecules found on Earth, including atmospheric nitrogen and water. The key lies in a combination of two kinds of catalysts, intermediate compounds which enable or speed up reactions without contributing to the final mixture, made especially for ammonia production, and which are driven by sunlight.
“This is the first successful example of photocatalytic ammonia production using atmospheric dinitrogen as a nitrogen source and water as a proton source, that also uses visible light energy and two kinds of molecular catalysts,” said Nishibayashi. “We used an iridium photocatalyst and another chemical called a tertiary phosphine which enabled photochemical activation of water molecules. The reaction efficiencies were higher than expected, compared to previous reports of visible light-driven photocatalytic ammonia formation.”
The thing about chemical reactions is, they don’t always happen as fast as you want, or in the way that you want. And to control the outcome, efficiency, timing and so on of a process, you need to involve additional components beyond just the raw ingredients. This is where the catalysts come in. Nishibayashi and his team utilized two catalysts for these experiments, one based on the transition metal molybdenum for the activation of dinitrogen and the other based on the transition metal iridium for the photoactivation of both tertiary phosphines and water. A third component called tertiary phosphines are also key to helping get the protons out of water molecules.
“When the iridium photocatalyst absorbs sunlight, its excited state can oxidize the tertiary phosphines. The oxidized tertiary phosphines then activate water molecules via formation of a chemical bond between the phosphine’s phosphorous atom and the water, yielding protons,” said Nishibayashi. “The molybdenum catalyst then enables nitrogen to bond with these protons to become ammonia. The use of water for producing dihydrogen or hydrogen atoms is one of the most important processes for achieving green ammonia production.”
The team managed to produce this reaction at a scale 10 times that of previous experiments, suggesting it’s ready for trials at larger scales, though there are still some issues that could improve the safety and effectiveness further. Some of the components such as the tertiary phosphines could be made using solar power or recycled from phosphine oxides. And while stable themselves, they may be toxic if ingested by people, so it would be ideal to find a responsible way to dispose of or recycle them.
“In plants, ammonia is formed by biological nitrogen fixation using cyanobacteria and is linked with photosynthesis,” said Nishibayashi. “Here, the electrons for the reaction are supplied by photosynthesis and protons are derived from water. Therefore, the findings of our recent study can be regarded as a successful example of the artificial photosynthesis of ammonia.”







