Obesity disrupts normal liver function in mice Comparison of mice at feeding and fasting shows biological regulation is reversed due to obesity Research news

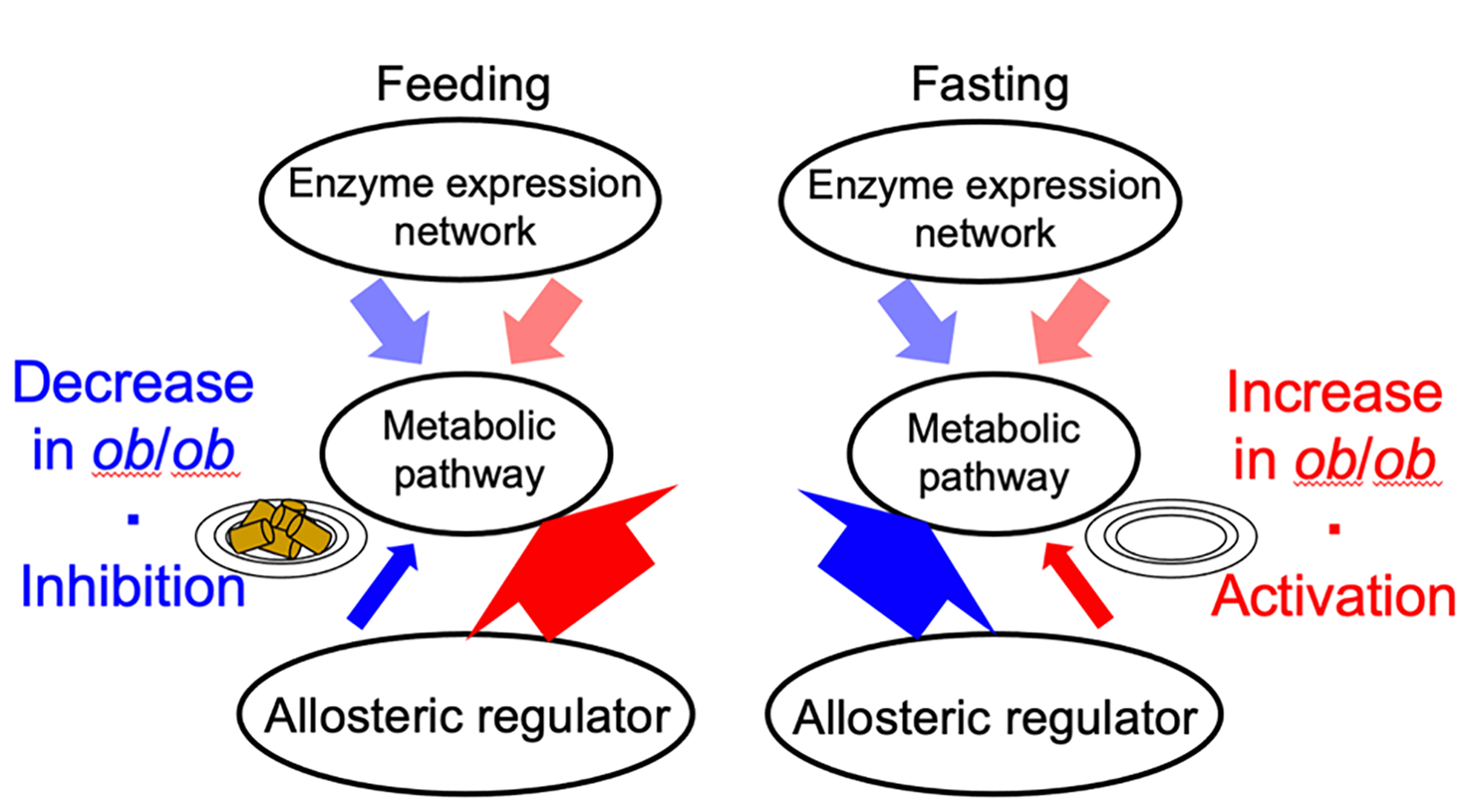
Allosteric response when feeding and fasting. An illustration of the results of the trans-omic analysis. Allosteric regulation, which controls metabolism, decreased in typical mice during feeding (thin blue arrow, left) and increased during fasting (thick blue arrow), but in obese (ob/ob) mice the reverse occurred (thick and thin red arrows). Enzyme expression did not change. © 2023 Shinya Kuroda
Your liver plays a vital role in your metabolism, the biological process which converts food into energy. We know that being overweight can negatively affect metabolic activity, but not exactly how. To better understand this, researchers compared the livers of mice which were a typical weight with mice which were obese. They were surprised to find that biological regulation of metabolic activity, after a period of feasting and fasting, was reversed between them. In typical mice, allosteric regulation (the process which controls metabolism) was inhibited during feeding and activated when fasting. However, in obese mice, allosteric regulation increased during feeding and decreased when fasting. Investigating the reasons behind this reversed biological behavior could help health professionals understand how obesity affects the body and the development of disease.
The World Obesity Federation (WOF) estimates that by 2035, over 4 billion people will be overweight or living with obesity. This may lead to a rise in obesity-related health conditions, such as heart disease, nonalcoholic fatty liver disease and Type 2 diabetes. Identifying the causes and effects of obesity, which is now understood to be a complex disease, is key for physicians looking to provide support and help people stay healthy.

Illustration of typical and obese ob/ob mice. Obese ob/ob mice eat excessively and are typically used for research into Type 2 diabetes. The mutated gene which causes increased appetite in ob/ob mice led to the discovery of the hormone leptin, which controls body fat by suppressing hunger and is lacking in the obese mice. © 2023 Shinya Kuroda
One known way that obesity can affect health is by impacting metabolism, the process by which our bodies take in, store and use energy from our food. Certain organs play key roles in this process, notably the liver. Not only is food processed there to provide energy, but it is one of the places where useful products at the end of the metabolic process are stored until we need them. To better understand the effects of obesity on the liver, researchers compared the livers of typical mice and obese mice after periods of feeding and fasting.
The team carried out trans-omics analysis, an approach where they gathered data on five sets of biological processes (multi-omics). They then combined these layers of data with information from biological databases to create a trans-omic network. This gave them an overview of how the different layers interacted. “We constructed a trans-omic network of metabolic reactions in the livers of mice that could feed freely. We then compared this with data we had previously gathered from mice that had fasted for 16 hours,” explained Professor Shinya Kuroda from the Graduate School of Science at the University of Tokyo. “While enzyme and allosteric regulation which controls metabolism was suppressed in typical mice during feeding, we were surprised to find that the reverse occurred in obese mice and that this activity increased.”
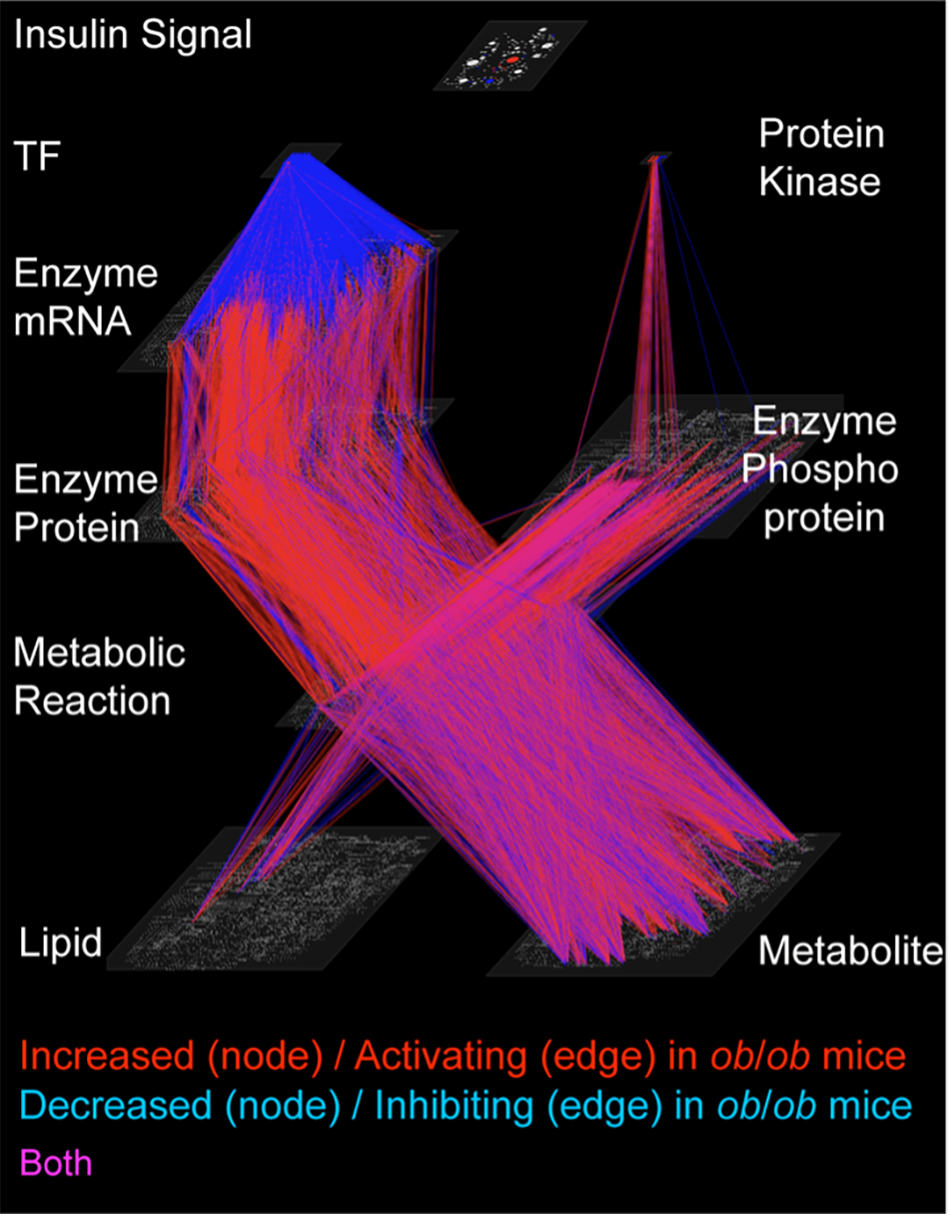
Trans-omic network. The omics included in this study were transcriptome (mRNAs), proteome (proteins), metabolome (metabolites), lipidome (lipids), phosphoproteomes and insulin-signaling molecules (phosphoproteins). By combining this data, the researchers constructed a trans-omic network. © 2023 Shinya Kuroda
When we eat, our liver builds up stores of energy which is then released as needed, a system known as metabolic homeostasis. However, the researchers saw that in obese mice this equilibrium became dysregulated, i.e., normal function was disrupted, indicating a potential breakdown of the system. This could lead to metabolic disorders such as tiredness, lack of energy and decreased appetite. By contrast, they saw that transcriptional regulation, a process which regulates metabolism and controls cell activity at a genetic level, did not change much between feeding and fasting. This means that, compared to allosteric regulation, it is more stable and less affected by what we eat.
The team noted that what they observed may not only be evidence of disruption within the liver alone, but a change to broader metabolic cycles throughout the body. “Obesity is a metabolic disease, so to understand it, it is important to construct a trans-omic network with metabolome (the complete set of small-molecule chemicals) at its center,” said Kuroda. “We are interested not only in the liver, but also how the products of metabolic reactions circulate between liver and muscle through the blood in obese mice, which is what we will be working on now.”
Papers
Yunfan Bai, Keigo Morita, Toshiya Kokaji, Atsushi Hatano, Satoshi Ohno, Riku Egami, Yifei Pan, Dongzi Li, Katsuyuki Yugi, Saori Uematsu, Hiroshi Inoue, Yuka Inaba, Yutaka Suzuku, Misaki Matsumoto, Masatomo Takahashi, Yoshihiro Izumi, Takeshi Bamba, Akiyoshi Hirayama, Tomoyoshi Soga, and Shinya Kuroda, "Trans-omic analysis reveals opposite metabolic dysregulation between feeding and fasting in liver associated with obesity," iScience: February 26, 2024, doi:10.1016/j.isci.2024.109121.
Link (Publication )
)
Related links
- Graduate School of Science

- Department of Biological Sciences

- [Article] Mapping connections between liver, muscle and metabolism in obesity

- [Article] Regulators of metabolism, diabetes identified with new technique

- [Press release] Obesity changes cell response to glucose, uses slower metabolic path in mouse liver

- [Press release] Different responses in individual cells give muscles more control

- [Press release] A subway map for diabetes






